
Interpretation:
The chemical formula for the given molecule should be identified.
Concept introduction:
According to the nomenclature, when two nonmetals are present in the given compound the name of the compound is given as follows, For example, HCl. According to the name of the compound, first give the name for the hydrogen followed by the second element, changing the ending of its name to –ide. Chlorine called as chloride. Therefore HCl is hydrogen chloride. Similarly, HI is hydrogen iodide. SiC is silicon carbide.
One pair of elements to form several different binary molecular compounds. In these cases, using of Greek prefixes to denote the number of atoms of each element present.
Greek prefixes are given below,

If prefix mono substituted is generally omitted for the first element. For example, SO2 is named sulfur dioxide, is not monosulfur dioxide. Moreover, only one atom in a prefix for the first element, no needs to mention mono or di etc.… In addition, for ease of pronunciation, we usually eliminate the last letter of a prefix that ends in o or a when naming an oxide. Thus, N2O5 is dinitrogen pentoxide, rather than dinitrogen pentaoxide.
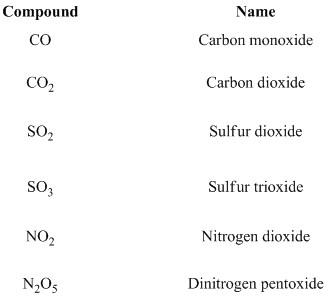
If any halogens are present in the molecule in suffix, the name of the halogens as follows.

Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Chemistry: Atoms First
- In an experiment, the viscosity of water was measured at different temperatures and the table was constructed from the data obtained. a) Calculate the activation energy of viscous flow (kJ/mol). b) Calculate the viscosity at 30°C. T/°C 0 20 40 60 80 η/cpoise 1,972 1,005 0,656 0,469 0,356arrow_forwardDon't used Ai solutionarrow_forwardLet's see if you caught the essentials of the animation. What is the valence value of carbon? a) 4 b) 2 c) 8 d) 6arrow_forward
- A laser emits a line at 632.8 nm. If the cavity is 12 cm long, how many modes oscillate in the cavity? How long does it take for the radiation to travel the entire cavity? What is the frequency difference between 2 consecutive modes?(refractive index of the medium n = 1).arrow_forwardA laser emits a line at 632.8 nm. If the cavity is 12 cm long, how many modes oscillate in the cavity? How long does it take for the radiation to travel the entire cavity? What is the frequency difference between 2 consecutive modes?(refractive index of the medium n = 1).arrow_forwardThe number of microstates corresponding to each macrostate is given by N. The dominant macrostate or configuration of a system is the macrostate with the greatest weight W. Are both statements correct?arrow_forward
- For the single step reaction: A + B → 2C + 25 kJ If the activation energy for this reaction is 35.8 kJ, sketch an energy vs. reaction coordinate diagram for this reaction. Be sure to label the following on your diagram: each of the axes, reactant compounds and product compounds, enthalpy of reaction, activation energy of the forward reaction with the correct value, activation energy of the backwards reaction with the correct value and the transition state. In the same sketch you drew, after the addition of a homogeneous catalyst, show how it would change the graph. Label any new line "catalyst" and label any new activation energy.arrow_forwardHow many grams of C are combined with 3.75 ✕ 1023 atoms of H in the compound C5H12?arrow_forwarde. f. CH3O. יון Br NaOCH3 OCH 3 Br H₂Oarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


