
(a)
Interpretation:
The electron configuration of silicon needs to be written and the orbital diagram of silicon needs to be drawn applying the Pauli Exclusion Principle, the Aufbau principle, and Hund's rule.
Concept introduction:
- Aufbau Principle states that in the ground state electrons of an atom fill the orbital with lowest energy first and upcoming electrons are filled in the order of increasing their energies.
- Hund's rule states that pairing of electrons do not occurs until each and every orbital in a subshell is singly filled with electrons having same spin.
- Pauli Exclusion Principle states that in an atom no two electrons present in a same orbital having same spin.
(a)
Answer to Problem 29SSC
Silicon
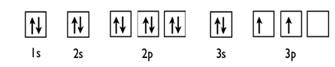
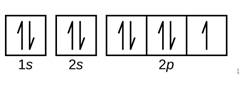
Explanation of Solution
Given: Silicon atom
An orbital diagram refers as the arrangement of the electrons in an atom. In the orbital diagram, the each orbital is shown as square and in a sublevel they are represented next to each other in a horizontal way.
Silicon
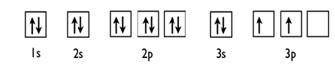
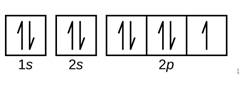
(b)
Interpretation:
The electron configuration of fluorine needs to be written and the orbital diagram of Krypton needs to be drawn applying the Pauli Exclusion Principle, the Aufbau principle, and Hund's rule.
Concept introduction:
- Aufbau Principle states that in the ground state electrons of an atom fill the orbital with lowest energy first and upcoming electrons are filled in the order of increasing their energies.
- Hund's rule states that pairing of electrons do not occurs until each and every orbital in a subshell is singly filled with electrons having same spin.
- Pauli Exclusion Principle states that in an atom no two electrons present in a same orbital having same spin.
(b)
Answer to Problem 29SSC
Fluorine
Explanation of Solution
Given: Fluorine atom
An orbital diagram refers as the arrangement of the electrons in an atom. In the orbital diagram, the each orbital is shown as square and in a sublevel they are represented next to each other in a horizontal way.
Fluorine 1
(c)
Interpretation:
The electron configuration of calcium needs to be written and the orbital diagram of Krypton needs to be drawn applying the Pauli Exclusion Principle, the Aufbau principle, and Hund's rule.
Concept introduction:
- Aufbau Principle states that in the ground state electrons of an atom fill the orbital with lowest energy first and upcoming electrons are filled in the order of increasing their energies.
- Hund's rule states that pairing of electrons do not occurs until each and every orbital in a subshell is singly filled with electrons having same spin.
- Pauli Exclusion Principle states that in an atom no two electrons present in a same orbital having same spin.
(c)
Answer to Problem 29SSC
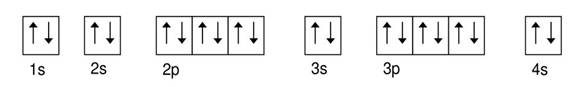
Calcium 3
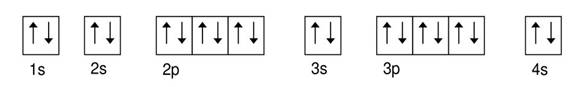
Explanation of Solution
Given: Calcium atom
An orbital diagram refers as the arrangement of the electrons in an atom. In the orbital diagram, the each orbital is shown as square and in a sublevel they are represented next to each other in a horizontal way.
Calcium
(d)
Interpretation:
The electron configuration of krypton needs to be written and the orbital diagram of Krypton needs to be drawn applying the Pauli Exclusion Principle, the Aufbau principle, and Hund's rule..
Concept introduction:
- Aufbau Principle states that in the ground state electrons of an atom fill the orbital with lowest energy first and upcoming electrons are filled in the order of increasing their energies.
- Hund's rule states that pairing of electrons do not occurs until each and every orbital in a subshell is singly filled with electrons having same spin.
- Pauli Exclusion Principle states that in an atom no two electrons present in a same orbital having same spin.
(d)
Answer to Problem 29SSC
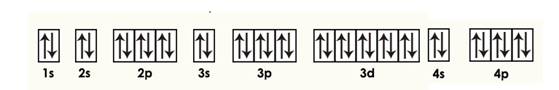
Krypton
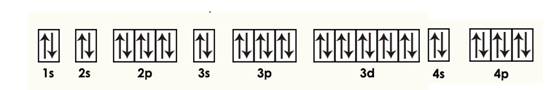
Explanation of Solution
Given: Krypton atom
An orbital diagram refers as the arrangement of the electrons in an atom. In the orbital diagram, the each orbital is shown as square and in a sublevel they are represented next to each other in a horizontal way.
Krypton
Chapter 5 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
General Chemistry: Principles and Modern Applications (11th Edition)
Chemistry: The Central Science (13th Edition)
Organic Chemistry (8th Edition)
Chemistry: A Molecular Approach
Introductory Chemistry (5th Edition) (Standalone Book)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





