
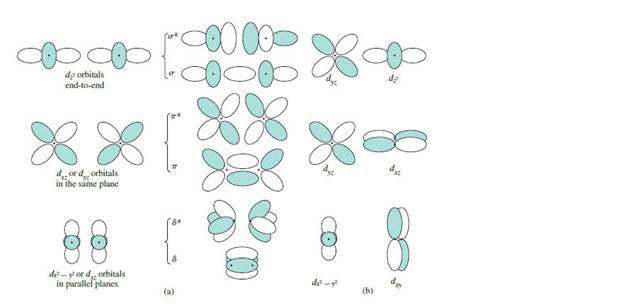
Repeat the process in the preceding example for the following orbital combinations, again usingcollinear z axes.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Inorganic Chemistry
Additional Science Textbook Solutions
Elementary Principles of Chemical Processes, Binder Ready Version
Organic Chemistry (9th Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
Chemistry: A Molecular Approach
Basic Chemistry
Principles of General, Organic, Biological Chemistry
- A nonmetallic element, R, burns brightly in air to give the oxide R4O10. If R is in Period 3, what is the ground-state valence-shell configuration of the atom?arrow_forwardrue or false? The hydrogen atom has a 3 orbital. Explain.arrow_forward• identify an orbital (as 1s, 3p, etc.) from its quantum numbers, or vice versa.arrow_forward
- Suppose that the spin quantum number did not exist, and therefore only one electron could occupy each orbital of a many-electron atom. Give the atomic numbers of the first three noble-gas atoms in this case.arrow_forwardFor the following pairs of orbitals, indicate which is lower in energy in a many-electron atom. (a) 3d or 4s (b) 4f or 3d (c) 2s or 2P d) 4f or 4darrow_forwardWhich of the following sets of quantum numbers correctly represents a 4p orbital? (a) n = 4, = 0, m = 1 (b) n = 4, = 1, m = 0 (c) n = 4, = 2, m = 1 (d) n = 4, = 1, m =2arrow_forward
- Which of the following subshells is allowed? O 4d5 O 1p? O 3p7 O 2d3 3f2arrow_forward5) Give the name and symbols for three ions that are isoelectronic with an unknown element whose electron configuration is [ Kr] 5s², 4d¹0, 5p6. 6) Give the box orbital diagram for the ground state configuration of barium. 7) Give the name and symbols for two ions that are isoelectronic with selenium ion. 8) Find the possible values for the quantum numbers of the highest energy electron meaning that outermost valence electron. a. Gallium b. Rubidium c. Sodiumarrow_forwardWhat are the sets of quantum numbers for the following hydrogen atomic orbitals? mi Orbital 4d-2 수 3po 5f1arrow_forward
- Sketch the following orbitals (including the x, y, andz axes): 1s, 2px, 3dxy, 3dz2.arrow_forwardAn atom easily loses two electrons to form the ion R2+. The element, which is in Period 6, forms the oxides RO and RO2. Give the orbital diagram for the ground state valence shell of the element that immediately follows R in the periodic table.arrow_forwardWhich ground-state atom has an electron configuration described by the following orbital diagram? 1L 1 11 11 11 11 1 1 4p [Ar] 4s 3d O Ge OP Se OK Tearrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





