
Concept explainers
Interpretation:
The molar mass of polychloroprene and its second osmotic virial coefficient has to be calculated.
Concept Introduction:
When large molecules dissolve to produce solutions that are not ideal solutions, it is assumed that the Van’t Hoff equation is the first term of the virial like expansion. The osmotic virial equation is,
Answer to Problem 5B.9P
The molar mass of polychloroprene is
Explanation of Solution
The equation used to determine the molar mass from osmotic pressure is,
Where,
The given data is,
The plot of
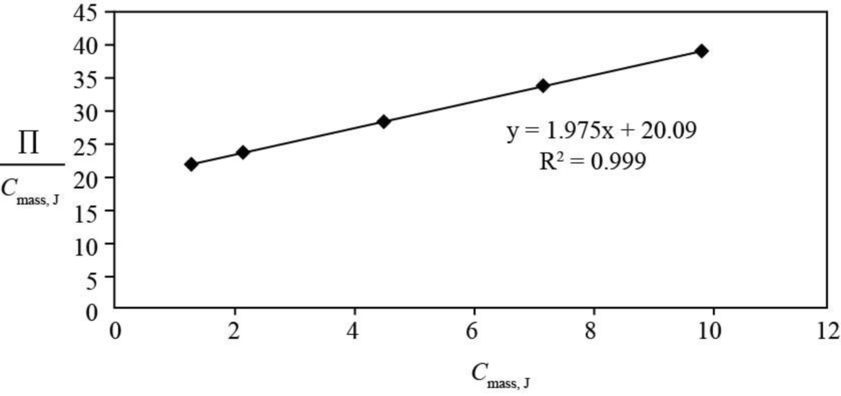
Figure 1
From the plot intercept is
The intercept of the plot of
The value of
The conversion of temperature,
The value of
The value of
The conversion of
Substitute the values of
The molar mass of polychloroprene is
The slope of the plot of
Substitute the value of
The conversion of
Hence, the conversion of
The value of second osmotic virial coefficient is
Want to see more full solutions like this?
Chapter 5 Solutions
ATKINS' PHYSICAL CHEMISTRY-ACCESS
- (12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- Don't used Ai solutionarrow_forwardIndicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are falsearrow_forward(f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
- 1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





