
(a)
Interpretation:
Atomic percent of
Concept Introduction:
Fick's first law of equation is as follows:
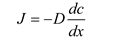
Answer to Problem 5.33P
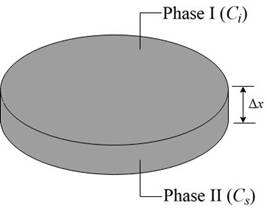
The concentration gradient in atomic percent
Explanation of Solution
The concentration gradient in atomic percent
Here,
The 1st phase composition in atomic percent
The 2nd phase composition in atomic percent is
The thickness of concentration gradient
Substitute the above value in the equaion
Hence, the concentration gradient in atomic percent
(b)
Interpretation:
The concentration gradient
Concept Introduction:
The concentration gradient can be calculated as follows:
Answer to Problem 5.33P
The value of concentration gradient in atomic
Explanation of Solution
Calculate the weight
The percentage of
The percentage of
Atomic weight of
Substitute the above value in equation
Substitute the above value of
Hence, the value of concentration gradient in atomic
Here minus sign indicates that flux is moving from higher concentration to lower concentration.
(c)
Interpretation:
The value of concentration gradient in
Concept Introduction:
The number of atoms of Zn per
Here, no. of atoms of
Answer to Problem 5.33P
The value of concentration gradient in
Explanation of Solution
Calculate the numbers of atoms per
Here no. of atoms of
The fraction of
Lattice parameter is
Substitute the above value in equation
Similarly,
Substitute the above value of
Hence, the value of concentration gradient in
Want to see more full solutions like this?
Chapter 5 Solutions
Essentials Of Materials Science And Engineering
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





