
Concept explainers
(a)
Interpretation:
Atomic orbitals which are used to form each
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Sigma (σ) bonds are the bonds in which shared hybrid orbital’s electron density are concentrated along the internuclear axis.
Pi (π) bonds are the bonds in which shared unhybridized orbital’s (p, d, etc) electron density are concentrated in above and below of the plane of the molecule.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
(a)
Explanation of Solution
In the marked carbon atom, one s and three p orbital hybridize forming four
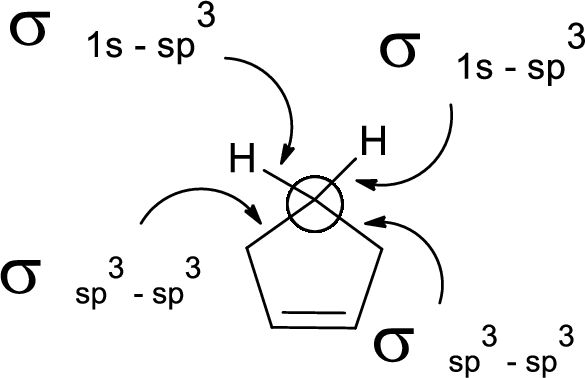
In the marked carbon atom, one s and two p orbital hybridize forming three
(b)
Interpretation:
Atomic orbitals which are used to form each
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Sigma (σ) bonds are the bonds in which shared hybrid orbital’s electron density are concentrated along the internuclear axis.
Pi (π) bonds are the bonds in which shared unhybridized orbital’s (p, d, etc) electron density are concentrated in above and below of the plane of the molecule.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
(b)
Explanation of Solution
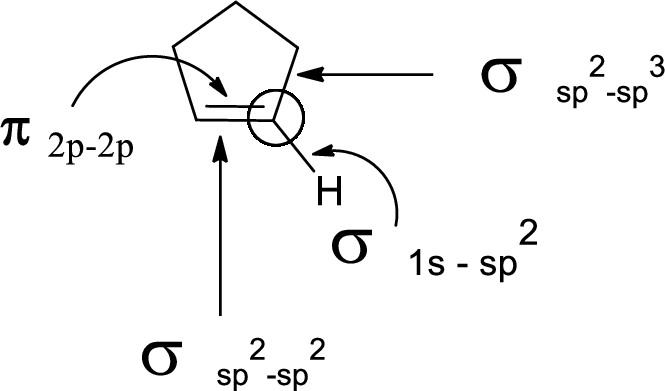
In the marked carbon atom, one s and two p orbital hybridize forming three
(c)
Interpretation:
Atomic orbitals which are used to form each
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Sigma (σ) bonds are the bonds in which shared hybrid orbital’s electron density are concentrated along the internuclear axis.
Pi (π) bonds are the bonds in which shared unhybridized orbital’s (p, d, etc) electron density are concentrated in above and below of the plane of the molecule.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
(c)
Explanation of Solution
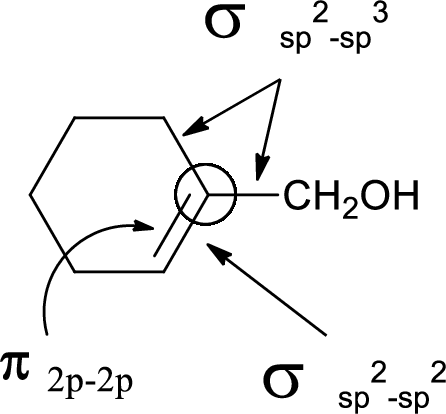
In the marked carbon atom, one s and two p orbital hybridize forming three
(d)
Interpretation:
Atomic orbitals which are used to form each
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Sigma (σ) bonds are the bonds in which shared hybrid orbital’s electron density are concentrated along the internuclear axis.
Pi (π) bonds are the bonds in which shared unhybridized orbital’s (p, d, etc) electron density are concentrated in above and below of the plane of the molecule.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
(d)
Explanation of Solution
In the marked carbon atom, one s and two p orbital hybridize forming three
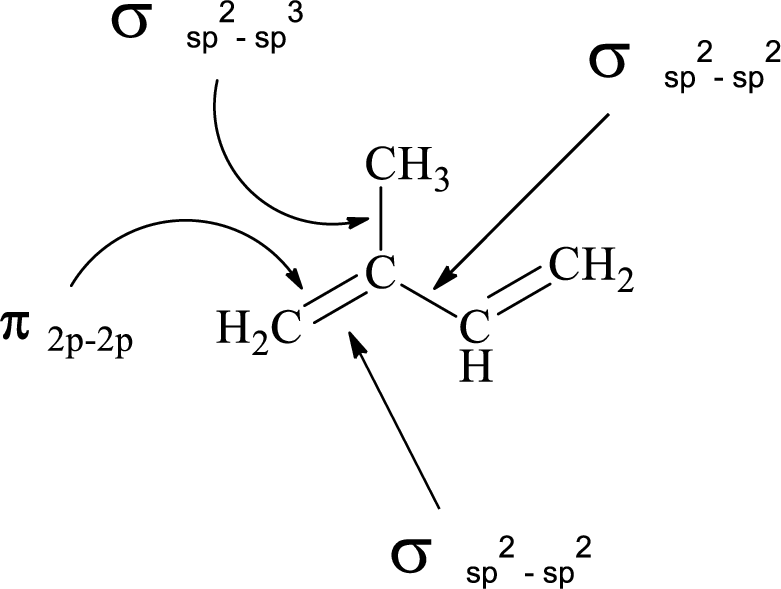
(e)
Interpretation:
Atomic orbitals which are used to form each
Concept Introduction:
Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
Sigma (σ) bonds are the bonds in which shared hybrid orbital’s electron density are concentrated along the internuclear axis.
Pi (π) bonds are the bonds in which shared unhybridized orbital’s (p, d, etc) electron density are concentrated in above and below of the plane of the molecule.
Geometry of different types of molecule with respect to the hybridizations are mentioned are mentioned below,
(e)
Explanation of Solution
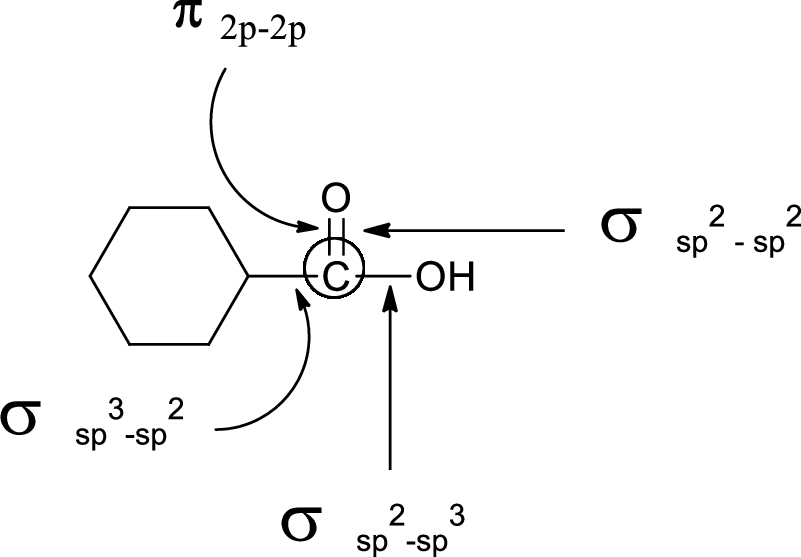
In the marked carbon atom, one s and one p orbital hybridize forming two
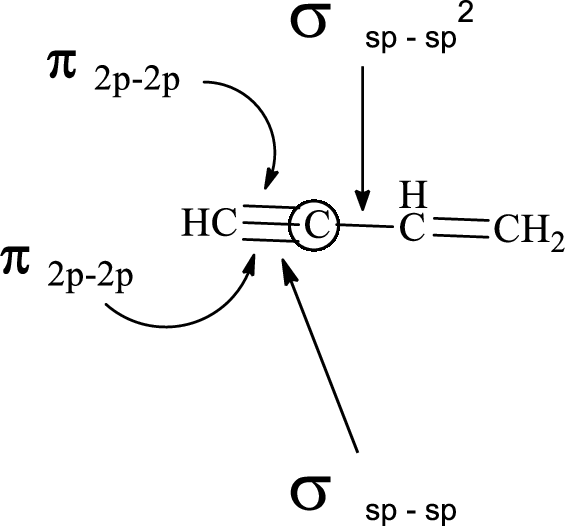
In the marked carbon atom, one s and two p orbital hybridize forming three
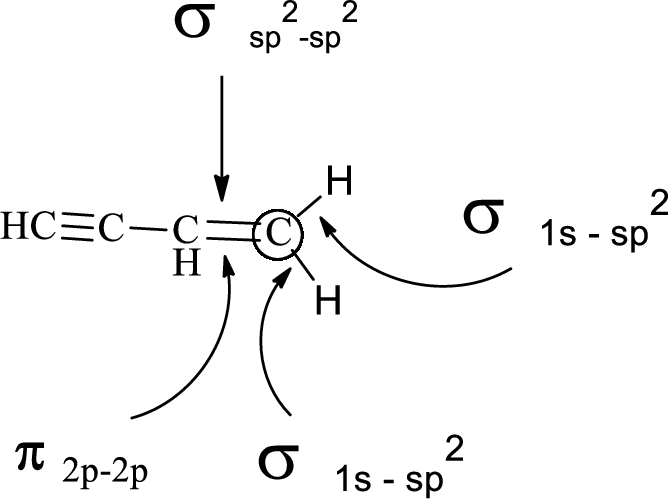
Want to see more full solutions like this?
Chapter 5 Solutions
OWL V2 with MindTap Reader and Student Solutions Manual eBook for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- For the molecular formula CH3NO: Draw a valid Lewis structure, showing all lone pairs of electrons. Draw a second, valid, and distinct Lewis structure from the first, again showing all lone pairs 1) 2) For each structure you drew, indicate which bond is most polar, and provide your reasoning. You should look up Pauling electronegativity values to answer this question. 3) For the bond you chose as the most polar in each structure, provide a molecular orbital description (not diagram!) of the bonding orbital overlaps. 4) For each structure you drew, indicate the hybridization of the carbon atom, the nitrogen atom, and the oxygen atom. 5) For the carbon atom in each structure, what is the hybridization? What is the shape around that carbon atom?arrow_forward3) How many electrons are delocalized in how many p-orbitals? Draw the most and second most stable resonance structures of the following compound. Order the structures by increasing stability. What is the partial charge distribution in the resonance hybrid?arrow_forwardThis is the shape of the sp hybrid carbons in this compound. tetrahedral trigonal planar linear square This is the General Formula of alkanes, where n is the number of C. CnH2n CnH2n-2 CnH2n+2 CnH2n-4 This class of organic compounds is saturated with only carbon-carbon single bonds. O Alkynes Alkenes Alkanes Arenes O Oarrow_forward
- Draw a three-dimensional representation of the valence orbitals and their bonding for compound CH2CHOH. Clearly show all valence electrons for each atom. Provide the following labels: atomic symbols, a code for each type of orbital used, a sigma bond, a pi bond and the molecular shape and approximate bond angles around the non-hydrogen atoms.arrow_forwardHow does the valence bond description of a carboncarbon double bond account for cis trans isomers?arrow_forwardMethyl isocyanate, CH3 -N= C = O, is used in the industrial synthesis of a type of pesticide and herbicide known as a carbamate. As a historical note, an industrial accident in Bhopal, India, in 1984 resulted in leakage of an unknown quantity of this chemical into the air. An estimated 200,000 people were exposed to its vapors, and over 2000 of these people died. Q.) Write a Lewis structure for methyl isocyanate and predict its bond angles. What is the hybridization of its carbonyl carbon? Of its nitrogen atom?arrow_forward
- 8. a) State the hybridization (sp°, sp', sp) of each carbon in this molecule, going left to right. HC b) Write "most" under the most stable alkene. Write "least" under the least stable alkene. c) Write "most" under the most stable cation. Write "least" under the least stable cation. CH2 CH3 CH3 CH3 CH3 H3C CHT HC H3C ONLY roactions we have studied this semester.arrow_forward: The three bonds in the carbon-carbon triple bond in H-C≡C-H are:a _______ bond formed from _______ orbitalsa _______ bond formed from _______ orbitalsa _______ bond formed from _______ orbitalsarrow_forwardPart G. Molecules with Oxygen and Nitrogen. Organic molecules often contain the elements oxygen and nitrogen. Construct a model with the molecular formula C5H12O where the oxygen is bonded to one hydrogen. When a hydrogen is bonded to an oxygen or nitrogen, you need to draw that bond in a bond-line structure. Draw the bond-line structure of this model. What is the molecular geometry of oxygen in your structure? Draw three more constitutional isomers with the molecular formula C5H12O where the oxygen is bonded to one hydrogen. Structure Structure Structure Draw three more constitutional isomers with the molecular formula C5H12O where the oxygen is bonded to two carbon atoms. Structure Structure Structurearrow_forward
- An alkene contains a double bond. Why is the C=C of an alkene rigid and unable to freely rotate? The C-C bond is very strong, and it is impossible to break it. The s bond becomes blocked by the hydrogen atoms. The sp2 hybrid orbitals of carbon take up too much room The p electrons are delocalized through the s bonds The 2p orbitals of the p bond overlap above and below the C-C plane to restrict rotation.arrow_forwardWhat are the molecular geometry and bond angle around a sp3 carbon atom?arrow_forwardDraw the complete p molecular orbital diagram (energy diagram) for allyl anion and fill up the orbitals with the appropriate number of p electrons. As well as the cation please ( "draw the resonance structure before starting the energy diagram")arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


