
Concept explainers
The photon energy and photon wavelength and the transition which absorb or emit the visible light.
Answer to Problem 41Q
The wavelength of transition from
Explanation of Solution
Given:
The first energy level is
The second energy level is
The third energy level is
Concept used:
Draw the energy level diagram for a photon.
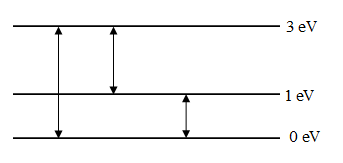
The possible transitions of an atom are shown in the above figure, the possible transitions are,
Write the expression for the Energy of the photon.
Here,
Rearrange Equation (1) for
Calculation:
For transition between
Substitute
The wavelength of transition from
For transition between
Substitute
The wavelength of transition from
For transition between
Substitute
The wavelength of transition from
Conclusion:
Thus, the wavelength of transition from
Want to see more full solutions like this?
Chapter 5 Solutions
UNIVERSE (LOOSELEAF):STARS+GALAXIES
- The first three energy levels of the fictitious element X are as shown.a. What wavelengths are observed in the absorption spectrum of element X? Give your answers in nm.b. State whether each of your wavelengths in part a corresponds to ultraviolet, visible, or infrared light.c. An electron with a speed of 1.4 × 106 m/s collides with an atom of element X. Shortly afterward, the atom emits a 1240 nm photon. What was the electron’s speed after the collision? Assume that, because the atom is so much more massive than the electron, the recoil of the atom is negligible.arrow_forwardAs shown the energy-level diagram of Element X.a. What is the ionization energy of Element X?b. An atom in the ground state absorbs a photon, then emits a photon with a wavelength of 1240 nm. What conclusion can you draw about the energy of the photon that was absorbed?c. An atom in the ground state has a collision with an electron, then emits a photon with a wavelength of 1240 nm. What conclusion can you draw about the initial kinetic energy of the electron?arrow_forwardAs shown the energy levels of a hypothetical atom.a. What minimum kinetic energy (in eV) must an electron have to collisionally excite this atom and cause the emission of a 620 nm photon? Explain.b. Can an electron with K = 6 eV cause the emission of 620 nm light from this atom? If so, what is the final kinetic energy of the electron? If not, why not?c. Can a 6 eV photon cause the emission of 620 nm light from this atom? Why or why not?d. Can a 7 eV photon cause the emission of 620 nm light from this atom? Why or why not?arrow_forward
- Calculate the energy of a 800 nm photon. b. Find the wavelength and frequency of a 80-MeV photon. Given that the maximum wavelength for photoelectric emission in tungsten is 230 nm, what wavelength of light must be used to eject electrons with a maximum energy of 1.6 eV?arrow_forwarda. How much energy is required to ionize a hydrogen atom containing an electron in the n=4 level? b. What wavelength of light contains enough energy in a single photon to ionize a hydrogen atom?arrow_forwardThe following diagram shows the complete set of orbitals of a hypothetical atom. The yellow circle represents the nucleus. Point D represents a location beyond the orbitals of this particular atom. Which of the following statements about an electron transitioning among the labeled points is TRUE? с D An electron transitioning from orbital A to orbital B will emit or absorb light with a longer wavelength than an electron transitioning from orbital B to orbital A. O The energy difference between orbitals B and C is bigger than that between orbitals A and B. To transition to a point between orbital A and B, an electron would need to absorb less energy than the difference between the energies of orbital A and B. An electron transitioning from orbital B orbital C would absorb green light. To transition from orbital C to orbital B, an electron must emit light.arrow_forward
- A hydrogen atom in an n=2 state absorbs a photon. Part a: What wavelength photons might be emitted by the atom following absorption?You should find 10 total possible wavelengths, with the shortest being around 100 nm and the largest being around 1900nm. What would the equation be in order to find these wavelengths?arrow_forwardIron has a magnetic moment of 2.22 Bohr magnetons per atom and a density of 7.87-103 kg.m-3. Calculate the expected magnetization of iron at 0 K and describe any assumptions that you have made. How would you expect this magnetization of iron to vary as temperature is increased. How does the number of Bohr magnetons per atom change from 0 K to 300 K. Why does a piece of iron typically not exhibit high magnetization at room temperature (unless it has been "magnetized")?arrow_forwardThe Pfund series in the hydrogen spectrum corresponds to transitions that have a final state of m=5. A. What are the wavelengths of the first three lines in this series? Express your answers in micrometers to three significant figures. Enter your answers in descending order separated by commas. B. What part of the electromagnetic spectrum are these lines in?arrow_forward
- For the purpose of this exercise, we consider the Earth as a blackbody at a temperature of 300K. a. Assuming that it is spherical with a radius equals to 6370 km, calculate the total amount energy emitted by the Earth (Hint: The total amount of energy emitted by a surface = amount of energy emitted per unit area x area of the surface). b. What wavelength range would you recommend to measure radiation emitted by the Earth using a satellite mounted sensor?arrow_forwardHelium was first discovered when astronomers viewed the spectrum from the Sun and could not associate absorption lines associated with any terrestrial element. One wavelength that was strongly absorbed was 587.5 nm (1 nm = 1.0×10-9 m). What is the energy associated with this wavelength of light? a. 1.06 eV (or 1.70×10-19 J) b. 0.943 eV (or 1.51×10-19 J) c. 2.11 eV (or 3.38×10-19 J) d. 0.472 eV (or 7.57×10-20 J)arrow_forwarda. Does a hot, thin gas emit a continuous spectrum, a bright line spectra with gaps between the lines, or a dark line spectra with all frequencies except the missing (absorbed) one? b. Why do we see dark line spectra when we look at stars? c. Both hydrogen and helium glow and absorb red. Are they the same frequency of red? d. A hot solid iron plate and a hot solid aluminum plate are the same temperature. Do the give off the same range of frequencies?arrow_forward
 University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning

