
Concept explainers
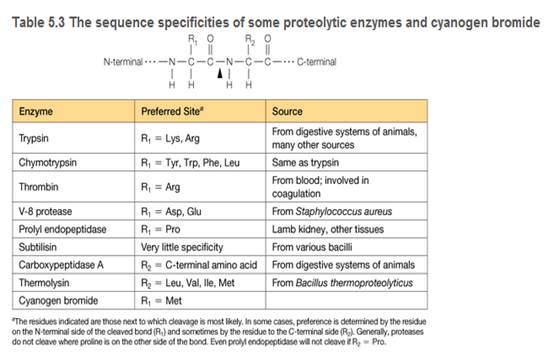
You are a summer intern in a clinical hematology lab. The lab director gives you a sample of a patient's blood proteins and asks you to characterize the thrombin in the sample. She also tells you that thrombin is a serine protease important in blood clotting (see Table 5.3), and this patient is a newborn with uncontrolled bleeding.
a. To characterize the thrombin in the sample, you must remove two proteins that interfere with the thrombin activity assay: cytochrome c and lactoglobin. You find some CM-cellulose (see Figure 5A.5) and a phosphate buffer (pH 6.4) on the shelf in your lab. You decide to load the protein sample onto a column of CM-cellulose equilibrated in the pH = 6.4 buffer. Predict the order of elution for the three proteins shown in the accompanying table. At pH = 6.4, which protein(s) do you predict will remain bound to the column?
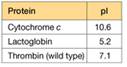
b. List two different ways you could change the buffer to elute the bound protein(s) and achieve proper separation of the proteins.
c. You are surprised to observe that the patient's thrombin flows through the CM-cellulose column at pH = 6.4 and does not bind. Confident in your technique, you suspect the patient's thrombin is different from wild-type thrombin. Using a different buffer system, you manage to purify some of the patient's thrombin, and you submit the purified sample for amino acid sequencing. The sequence analysis shows that the patient's thrombin contains a mutation in the enzyme active site. A lysine residue in the wild type has been mutated to an asparagine in the patient's thrombin. Does this mutation explain the anomalous CM- cellulose binding behavior you observed?
d. How many
e. Based on their side-chain structures, compare and contrast the potential of Lys and Asn to form noncovalent interactions. In other words, can each form H bonds and/or salt bridges and/or van der Waals contacts?
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Biochemistry: Concepts and Connections
- (a) Answer all sections (i)- (Iv). Below is given the structure of three anti-HIV drugs: OH Meo. OMe NH Ph Nevirapine Atazanavir Staudivine (i) Give the drug target and discuss the anti-HIV mechanism of each of these drugs. Vaccines are an attractive prophylactic treatment against both viral and bacterial diseases. A number of glycaconjugate vaccines using bacterial polysaccharide structures conjugated to protein carriers have been introduced into mass vaccination schemes with excellent results. What differences are there in the immune response to a glycoconjugate vaccine as compared to a polysaccharide vaccine? These glycoconjugate va nes has been used for 30 years without any change in their structures, while the composition of the flu vaccine, based on attenuated or killed virus particies, is changed from year to year. Discuss the reason behind this. (iv) Discuss why it has not been possible to develop a (part structure) vaccine against the HIV virus, based on either I) viral…arrow_forwardTrue or False: An IgM monomer can bind as many as 10 epitopes?arrow_forwardSickle-cell anemia Work out amino acid sequences.arrow_forward
- Glucose reacts slowly with hemoglobin and other proteins to form covalent compounds. Why is glucose reactive? What is the nature of the adduct formed?arrow_forwardBence-Jones protein is a protein with a molecular weight of approximately 150 kDa and is found in high concentration in the urine of Multiple Myeloma patients. Multiple Myeloma is a type of cancer in which lymphocytes multiply and break down bone marrow and bone. Which method would you prefer to show the Bence-Jones protein in the urine for the diagnosis of the disease? Explain the principle of your preferred method.arrow_forwardMonoclonal antibodies can be conjugated to an insoluble support by chemical methods. Explain how these antibody-bound beads can be exploited for protein purification.arrow_forward
- The Bence-Jones protein is a protein with a molecular weight of approximately 150 kDa and is found in high concentration in the urine of MulKpl Myeloma patients. MulKpl Myeloma is a type of cancer in which lymphocytes multiply and break down bone marrow and bone. Which method would you prefer to show the Bence-Jones protein in the urine for the diagnosis of the disease? Explain the principle of your preferred method.arrow_forwardDefine adenosine diphosphate (ADP)arrow_forwardWhat will be the effect of the following changes to the protein structure of hemoglobin and its function? A. Replacement of the proximal histidine residue at the 8thposition of the F helix by asparagine. B. A phenylalanine to proline mutation resulting in decreased cooperativity of the polypeptide chains. C. Replacement of a nonpolar amino acid in the interior of the protein to a hydrophilic amino acid.arrow_forward
- One of your patients, a six-year-old girl who suffers from Sickle cell anemia, an inherited blood disorder in which red blood cells are abnormally shaped and fragile, leading to a short supply of red blood cells. These abnormal cells can also get stuck in small vessels, which prevent blood flow, leading to fatigue, pain, and other severe complications. Patients with sickle cell anemia produce defective beta-globin due to a point mutation that causes the change of a single amino acid residue. This is an example of what type of mutation? nonsense mutation missense mutation frameshift mutation deletion mutationarrow_forwardYou are analyzing the peptide ala-ile-glu-lys-phe-val- tyr-cys. If you treat the peptide with chymotrypsin, which technique would be best to separate the fragments? O a. ultracentrifugation O b. affinity chromatography O c. dialysis d. gel filtration chromatography O e. ion exchange chromatography What is an advantage of NMR spectroscopy over x-ray crystallography in studying protein structure?arrow_forward(e) Two ADP agonists (drugs) were also found to bind to platelets: 2-methylthio-ADP bound with a Kp of 7 µM and 2-(3-aminopropylthio)-ADP bound with a Kp of 200 µM. Can these drugs effectively compete with ADP for binding to platelets? Explain your answer.arrow_forward
