
Interpretation:
An efficient synthesis for the given transformation has to be predicted. The given reaction is shown as,
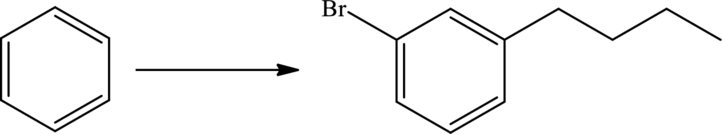
Concept Introduction:
Deactivators are electron withdrawing groups attached to the benzenes that have either positive charge or an atom with high electronegativity. They are meta directors.
Activators are electron donating groups attached to the benzenes that have either electron density that is able to push into benzene ring or a lone pair of electrons. They are ortho-para directing.
Halogens are deactivators that are ortho-para directing.
Bromination: A reaction in which a bromine atom is introduced to the compound.
Friedel-Crafts Acylation: This Lewis acid-catalyzed electrophilic
The reduction of
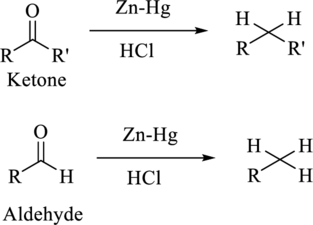
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
- If a high molecular weight linear polyethylene is chlorinated by inducing the substitution of chlorine atoms by hydrogen, if 5% of all hydrogen atoms are replaced, what approximate percentage of chlorine by weight would the product have?arrow_forwardO Macmillan Learning Chemistry: Fundamentals and Principles Davidson presented by Macmillan Learning Poly(ethylene terephthalate), known as PET or industrially as Dacron, is a polyester synthesized through a condensation reaction between two bifunctional monomers. The monomers, ethylene glycol and terepthalic acid, are given. Add bonds and remove atoms as necessary to show the structure of a two repeat unit portion of a longer polymer chain of PET. You may need to zoom out to see the complete structure of all four monomer units. Select Draw / || | C H 0 3 © Templates More ° ° ° || C CC - OH HO OH HOC - C Erase CC OH HO C C 〃 C H₂ Q2Qarrow_forwardc) + H₂Oarrow_forward
- 으 b) + BF. 3 H2Oarrow_forwardQ4: Draw the product of each Lewis acid-bas reaction. Label the electrophile and nucleophile. b) S + AICI 3 + BF 3arrow_forwardQ1 - What type(s) of bonding would be expected for each of the following materials: solid xenon, calcium fluoride (CaF2), bronze, cadmium telluride (CdTe), rubber, and tungsten? Material solid xenon CaF2 bronze CdTe rubber tungsten Type(s) of bonding Q2- If the atomic radius of lead is 0.175 nm, calculate the volume of its unit cell in cubic meters.arrow_forward
- Determine the atomic packing factor of quartz, knowing that the number of Si atoms per cm3 is 2.66·1022 and that the atomic radii of silicon and oxygen are 0.038 and 0.117 nm.arrow_forwardUse the following data for an unknown gas at 300 K to determine the molecular mass of the gas.arrow_forward2. Provide a complete retrosynthetic analysis and a complete forward synthetic scheme to make the following target molecule from the given starting material. You may use any other reagents necessary. Brarrow_forward
- 146. Use the following data for NH3(g) at 273 K to determine B2p (T) at 273 K. P (bar) 0.10 0.20 0.30 0.40 0.50 0.60 (Z -1)/10-4 1.519 3.038 4.557 6.071 7.583 9.002 0.70 10.551arrow_forward110. Compare the pressures given by (a) the ideal gas law, (b) the van der Waals equation, and (c) the Redlic-Kwong equation for propane at 400 K and p = 10.62 mol dm³. The van der Waals parameters for propane are a = 9.3919 dm6 bar mol-2 and b = 0.090494 dm³ mol−1. The Redlich-Kwong parameters are A = 183.02 dm bar mol-2 and B = 0.062723 dm³ mol-1. The experimental value is 400 bar.arrow_forwardResearch in surface science is carried out using stainless steel ultra-high vacuum chambers with pressures as low as 10-12 torr. How many molecules are there in a 1.00 cm3 volume at this pressure and at a temperature of 300 K? For comparison, calculate the number of molecules in a 1.00 cm3 volume at atmospheric pressure and room temperature. In outer space the pressure is approximately 1.3 x 10-11 Pa and the temperature is approximately 2.7 K (determined using the blackbody radiation of the universe). How many molecules would you expect find in 1.00 cm3 of outer space?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
