
Concept explainers
(a)
Interpretation:
Enatiomers for the given compound has to be drawn using perspective formula.
Concept Introduction:
Perspective formulas show the 3D array of atoms in which the solid wedges indicate bonds projecting above the plane of the drawing and wedge shaded with parallel line indicate bonds projecting below the plane of drawing.
Example:
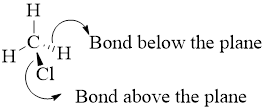
Enantiomers: These are stereoisomers that are not superimposable mirror images of each other and the configurations at all stereo genic centers are exactly opposite.
Enantiomer can be drawn by replacing the wedge with a dash from the perspective formula of a compound.
Chiral center: A chiral center is defined as the tetrahedral carbon atom in an organic molecule that is connected to four non-identical groups/substituents. It is sometimes known as a stereo genic center.
An achiral carbon is a carbon having two or more identical groups around it.
(b)
Interpretation:
Enatiomers for the given compound has to be drawn using perspective formula.
Concept Introduction:
Perspective formulas show the 3D array of atoms in which the solid wedges indicate bonds projecting above the plane of the drawing nad wedge shaded with parallel line indicate bonds projecting below the plane of drawing.
Example:
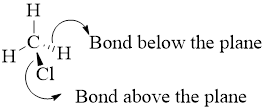
Enantiomers: These are stereoisomers that are not superimposable mirror images of each other and the configurations at all stereo genic centers are exactly opposite.
Enantiomer can be drawn by replacing the wedge with a dash from the perspective formula of a compound.
Chiral center: A chiral center is defined as the tetrahedral carbon atom in an organic molecule that is connected to four non-identical groups/substituents. It is sometimes known as a stereo genic center.
An achiral carbon is a carbon having two or more identical groups around it.
(c)
Interpretation:
Enatiomers for the given compound has to be drawn using perspective formula.
Concept Introduction:
Perspective formulas show the 3D array of atoms in which the solid wedges indicate bonds projecting above the plane of the drawing nad wedge shaded with parallel line indicate bonds projecting below the plane of drawing.
Example:
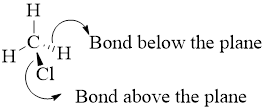
Enantiomers: These are stereoisomers that are not superimposable mirror images of each other and the configurations at all stereo genic centers are exactly opposite.
Enantiomer can be drawn by replacing the wedge with a dash from the perspective formula of a compound.
Chiral center: A chiral center is defined as the tetrahedral carbon atom in an organic molecule that is connected to four non-identical groups/substituents. It is sometimes known as a stereo genic center.
An achiral carbon is a carbon having two or more identical groups around it.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Essential Organic Chemistry (3rd Edition)
- For the compound below please choose the correct set of chair and flipped chair conformations:arrow_forwardDraw the enantiomer of the following compound: НО HO OH Z-I N.arrow_forwardDraw a three-dimensional structure of a chiral compound with the molecular formula of C4H4Cl2 that does not have a stereogenic carbon. In addition, draw the enantiomer of this compound.arrow_forward
- Which of the following is an enantiomer of the compound shown below? CH3 HC CI H O CH3 H--C1 H H C1-|-CH3 I H Cl H--H CH3 A and B It does not have an enantiomer.arrow_forwardClassify the following pair of compounds as the same compound, enantiomers, diastereomers, constitutional isomers, or not isomeric. Also, select the correct IUPAC name, including the correct (R) or (S) designation, for each. H ÷ ||I The correct IUPAC names are: || J k CI same compound enantiomers diastereomers constitutional isomers not isomeric Compound I: (2R, 3R)-2,3-dichloropentane, Compound II: (2R, 3S)-2,3-dichloropentane Compound I: (2S, 3S)-2,3-dichloropentane, Compound II: (2S, 3S)-2,3-dichloropentane Compound I: (2S, 3R)-2,3-dichloropentane, Compound II: (2S, 3R)-2,3-dichloropentane Compound I: (2R, 3R)-2,3-dichloropentane, Compound II: (2R, 3R)-2,3-dichloropentanearrow_forwardFor each of the following compounds, draw three dimensional (perspective) diagrams of the enantiomeric pair. Indicate any pairs that would be configurationally stable (i.e. separable) at room temperaturearrow_forward
- Determine whether the following pair of drawings represents: enantiomers, diastereomers, the same compound, or constitutional isomers. Hz N-arrow_forwardFor the compound NEGATRON, how many are the expected stereoisomers? Using Fischer projections, draw the enantiomer and diastereomers of the said compound. CH3 он NEGATRONarrow_forwardDraw the pair of enantiomers of this structure using three-dimensional representation.arrow_forward
- Draw the pair of enantiomersarrow_forwardKeppra is a medication used to treat epileptic seizures. Draw the enantiomer of the molecule shown below. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers, where applicable. & Sm NH₂ 'N O Drawing aarrow_forwardThere are 4 optionsarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


