
EBK THERMODYNAMICS: AN ENGINEERING APPR
8th Edition
ISBN: 8220100257056
Author: CENGEL
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.5, Problem 65P
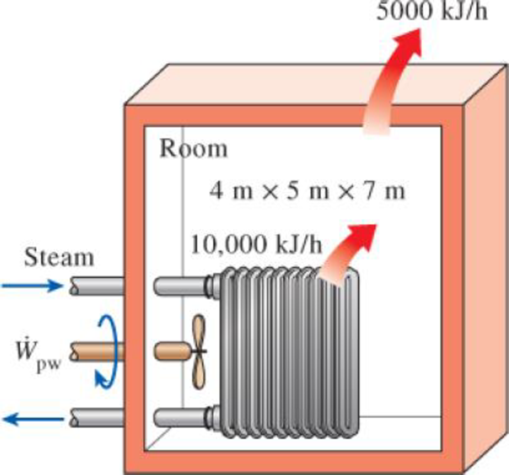
A 4-m × 5-m × 7-m room is heated by the radiator of a steam-heating system. The steam radiator transfers heat at a rate of 10,000 kJ/h, and a 100-W fan is used to distribute the warm air in the room. The rate of heat loss from the room is estimated to be about 5000 kJ/h. If the initial temperature of the room air is 10°C, determine how long it will take for the air temperature to rise to 20°C. Assume constant specific heats at room temperature.
FIGURE P4–64
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Water is being heated in a close pan on top of a range while being stirred by a paddle wheel. During the process, 30kj of heat are transferred to the water, and 5kj of heat is lost to the surrounding air. The paddle-wheel work amounts to 500 N*m. Determine the final energy of the system if its initial energy is 10kj.
5. A 4-m x 5-m x 7-m room is heated by the radiator of a steam-heating system. The
steam radiator transfers heat at a rate of 10,000 kJ/h, and a 100-W fan is used to distribute the warm air in the room. The rate of heat loss from the room is estimated to be about 5000 kJ/h. If the initial temperature of the room air is 10°C, determine
how long it will take for the air temperature to rise to 20oC. Assume constant specific
heats at room temperature where C of AIR = 0.768kJ/kg-K. draw a figure also, and explain each step by step solution.
5. A 4-m x 5-m x 7-m room is heated by the radiator of a steam-heating system. The steam radiator transfers heat at a rate of 10,000 kJ/h, and a 100-W fan is used to distribute the warm air in the room. The rate of heat loss from the room is estimated to be about 5000 kJ/h. If the initial temperature of the room air is 10°C, determine gow long it will take for the air temperature to rise to 20oC. Assume constant specific heats at room temperature where CAIR = 0.768kJ/kg-K. Draw a figure or FBD that will support the problem. Explain each step by step formula.
Chapter 4 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
Ch. 4.5 - An ideal gas at a given state expands to a fixed...Ch. 4.5 - Nitrogen at an initial state of 300 K, 150 kPa,...Ch. 4.5 - 4–3 The volume of 1 kg of helium in a...Ch. 4.5 - 4–4E Calculate the total work, in Btu, for process...Ch. 4.5 - 4–5 A piston–cylinder device initially contains...Ch. 4.5 - A pistoncylinder device with a set of stops...Ch. 4.5 - 4–7 A piston–cylinder device initially contains...Ch. 4.5 - 4–8 A mass of 5 kg of saturated water vapor at 300...Ch. 4.5 - 1 m3 of saturated liquid water at 200C is expanded...Ch. 4.5 - A gas is compressed from an initial volume of 0.42...
Ch. 4.5 - A mass of 1.5 kg of air at 120 kPa and 24C is...Ch. 4.5 - During some actual expansion and compression...Ch. 4.5 - 4–14 A frictionless piston–cylinder device...Ch. 4.5 - Prob. 15PCh. 4.5 - During an expansion process, the pressure of a gas...Ch. 4.5 - A pistoncylinder device initially contains 0.4 kg...Ch. 4.5 - 4–19E Hydrogen is contained in a piston–cylinder...Ch. 4.5 - A pistoncylinder device contains 0.15 kg of air...Ch. 4.5 - 1 kg of water that is initially at 90C with a...Ch. 4.5 - Prob. 22PCh. 4.5 - An ideal gas undergoes two processes in a...Ch. 4.5 - A pistoncylinder device contains 50 kg of water at...Ch. 4.5 - Prob. 26PCh. 4.5 - 4–27E A closed system undergoes a process in which...Ch. 4.5 - A rigid container equipped with a stirring device...Ch. 4.5 - A 0.5-m3rigid tank contains refrigerant-134a...Ch. 4.5 - A 20-ft3 rigid tank initially contains saturated...Ch. 4.5 - Prob. 31PCh. 4.5 - Prob. 32PCh. 4.5 - Prob. 33PCh. 4.5 - An insulated pistoncylinder device contains 5 L of...Ch. 4.5 -
4–35 A piston–cylinder device initially...Ch. 4.5 - Prob. 37PCh. 4.5 - A 40-L electrical radiator containing heating oil...Ch. 4.5 - Steam at 75 kPa and 8 percent quality is contained...Ch. 4.5 - Prob. 40PCh. 4.5 - An insulated tank is divided into two parts by a...Ch. 4.5 - Is the relation u = mcv,avgT restricted to...Ch. 4.5 - Is the relation h = mcp,avgT restricted to...Ch. 4.5 - Is the energy required to heat air from 295 to 305...Ch. 4.5 - A fixed mass of an ideal gas is heated from 50 to...Ch. 4.5 - A fixed mass of an ideal gas is heated from 50 to...Ch. 4.5 - A fixed mass of an ideal gas is heated from 50 to...Ch. 4.5 - Prob. 49PCh. 4.5 - What is the change in the enthalpy, in kJ/kg, of...Ch. 4.5 - Prob. 51PCh. 4.5 - Prob. 52PCh. 4.5 - Prob. 53PCh. 4.5 - Determine the internal energy change u of...Ch. 4.5 - Prob. 55PCh. 4.5 - Prob. 56PCh. 4.5 - Is it possible to compress an ideal gas...Ch. 4.5 - A 3-m3 rigid tank contains hydrogen at 250 kPa and...Ch. 4.5 - A 10-ft3 tank contains oxygen initially at 14.7...Ch. 4.5 - 4–60E A rigid tank contains 10 Ibm of air at 30...Ch. 4.5 - 4–61E Nitrogen gas to 20 psia and 100°F initially...Ch. 4.5 - An insulated rigid tank is divided into two equal...Ch. 4.5 - 4–63 A 4-m × 5-m × 6-m room is to be heated by a...Ch. 4.5 - 4-64 A student living in a 3-m × 4-m × 4-m...Ch. 4.5 - A 4-m 5-m 7-m room is heated by the radiator of...Ch. 4.5 - 4–66 Argon is compressed in a polytropic process...Ch. 4.5 - An insulated pistoncylinder device contains 100 L...Ch. 4.5 - 4–68 A spring-loaded piston-cylinder device...Ch. 4.5 - An ideal gas contained in a pistoncylinder device...Ch. 4.5 - Air is contained in a variable-load pistoncylinder...Ch. 4.5 - Prob. 71PCh. 4.5 - Prob. 72PCh. 4.5 - Prob. 74PCh. 4.5 - Prob. 75PCh. 4.5 - Prob. 76PCh. 4.5 - 4–77 Air is contained in a piston-cylinder device...Ch. 4.5 - A pistoncylinder device contains 4 kg of argon at...Ch. 4.5 - The state of liquid water is changed from 50 psia...Ch. 4.5 - During a picnic on a hot summer day, all the cold...Ch. 4.5 - Consider a 1000-W iron whose base plate is made of...Ch. 4.5 - Stainless steel ball bearings ( = 8085 kg/m3 and...Ch. 4.5 - In a production facility, 1.6-in-thick 2-ft 2-ft...Ch. 4.5 - Prob. 84PCh. 4.5 - An electronic device dissipating 25 W has a mass...Ch. 4.5 - Prob. 87PCh. 4.5 - 4–88 In a manufacturing facility, 5-cm-diameter...Ch. 4.5 - Prob. 89PCh. 4.5 - Is the metabolizable energy content of a food the...Ch. 4.5 - Is the number of prospective occupants an...Ch. 4.5 - Prob. 92PCh. 4.5 - Prob. 93PCh. 4.5 - Consider two identical 80-kg men who are eating...Ch. 4.5 - A 68-kg woman is planning to bicycle for an hour....Ch. 4.5 - A 90-kg man gives in to temptation and eats an...Ch. 4.5 - A 60-kg man used to have an apple every day after...Ch. 4.5 - Consider a man who has 20 kg of body fat when he...Ch. 4.5 - Consider two identical 50-kg women, Candy and...Ch. 4.5 - Prob. 100PCh. 4.5 - Prob. 101PCh. 4.5 - Prob. 102PCh. 4.5 - Prob. 103PCh. 4.5 - Prob. 104PCh. 4.5 - Prob. 105PCh. 4.5 - Prob. 106PCh. 4.5 - Prob. 107RPCh. 4.5 - Consider a pistoncylinder device that contains 0.5...Ch. 4.5 - Air in the amount of 2 lbm is contained in a...Ch. 4.5 - Air is expanded in a polytropic process with n =...Ch. 4.5 - Nitrogen at 100 kPa and 25C in a rigid vessel is...Ch. 4.5 - Prob. 112RPCh. 4.5 - Prob. 113RPCh. 4.5 - Prob. 114RPCh. 4.5 - 4–115 A mass of 12 kg of saturated...Ch. 4.5 - Prob. 116RPCh. 4.5 - Prob. 117RPCh. 4.5 - Prob. 118RPCh. 4.5 - Prob. 119RPCh. 4.5 - Prob. 120RPCh. 4.5 - Prob. 121RPCh. 4.5 - Prob. 122RPCh. 4.5 - Prob. 123RPCh. 4.5 - Prob. 124RPCh. 4.5 - Prob. 125RPCh. 4.5 - Prob. 126RPCh. 4.5 - Prob. 127RPCh. 4.5 - Prob. 128RPCh. 4.5 - A well-insulated 3-m 4m 6-m room initially at 7C...Ch. 4.5 - Prob. 131RPCh. 4.5 - Prob. 133RPCh. 4.5 - Prob. 134RPCh. 4.5 - An insulated pistoncylinder device initially...Ch. 4.5 - Prob. 137RPCh. 4.5 - Prob. 138RPCh. 4.5 - A pistoncylinder device initially contains 0.35 kg...Ch. 4.5 - Prob. 140RPCh. 4.5 - 4–141 One kilogram of carbon dioxide is compressed...Ch. 4.5 - Prob. 142RPCh. 4.5 - Prob. 143RPCh. 4.5 - Prob. 144FEPCh. 4.5 - A 3-m3 rigid tank contains nitrogen gas at 500 kPa...Ch. 4.5 - Prob. 146FEPCh. 4.5 - A well-sealed room contains 60 kg of air at 200...Ch. 4.5 - Prob. 148FEPCh. 4.5 - A room contains 75 kg of air at 100 kPa and 15C....Ch. 4.5 - A pistoncylinder device contains 5 kg of air at...Ch. 4.5 - Prob. 151FEPCh. 4.5 - Prob. 152FEPCh. 4.5 - A 2-kW electric resistance heater submerged in 5...Ch. 4.5 - 1.5 kg of liquid water initially at 12C is to be...Ch. 4.5 - An ordinary egg with a mass of 0.1 kg and a...Ch. 4.5 - 4–156 An apple with an average mass of 0.18 kg and...Ch. 4.5 - A 6-pack of canned drinks is to be cooled from 18C...Ch. 4.5 - An ideal gas has a gas constant R = 0.3 kJ/kgK and...Ch. 4.5 - Prob. 159FEPCh. 4.5 - Prob. 161FEP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Steam flows steadily through a turbine at a rate of 45,000 lbm/h. It enters at 1000 psi and 900°F and leaves at 5 psi as saturated vapor. If the power generated by the turbine is 4 MW, determine the rate of heat loss from the steam.arrow_forwardThe ducts of an air heating system pass through an unheated area. As a result of heat losses, the temperature of the air in the duct drops by 4°C. If the mass flow rate of air is 120 kg/min, determine the rate of heat loss from the air to the cold environmentarrow_forward3- A 5-m x 6-m x 8-m room is to be heated by an electrical resistance heater placed in a short duct in the room. Initially, the room is at 15°C, and the local atmospheric pressure is 98 kPa. The room is losing heat steadily to the outside at a rate of 200 kJ/min. A 200-W fan circulates the air steadily through the duct and the electric heater at an average mass flow rate of 50 kg/min. The duct can be assumed to be adiabatic, and there is no air leaking in or out of the room. If it takes 15 minutes for the room air to reach an average temperature of 25°C, find (a) the power rating of the electric heater and (b) the temperature rise that the air experiences each time it passes through the heater.arrow_forward
- 5-36 Steam enters a nozzle at 400°C and 800 kPa with a velocity of 10 m/s and leaves at 375°C and 400 kPa while losing heat at a rate of 25 kW. For an inlet area of 800 cm², determine the velocity and the volume flow rate of the steam at the nozzle exit. 400°C 800 kPa 10 m/s Steam 375°C 400 kPaarrow_forwardA piston-cylinder device as shown in Figure 1-7, initially contains 0.75 kg of refrigerant- 134a at 120 kPa and 20°C. Heat is now transferred to the refrigerant from a source at 150°C, and the piston, which is resting on a set of stops, starts moving when the pressure inside reaches 140 kPa. Heat transfer continues until the temperature reaches 90°C. Assuming the surroundings to be at 25°C and 100 kPa, determine: (a) the work done, (b) the heat transfer, (c) the exergy destroyed, (d) the second law efficiency of this process. Environment 100 kPa 25°C R-134a R-134a 0.75 kg 0.75 kg Нeat 120 kPa 140 kPa 20°C 90°C Initial State Heat Source Final State 150°C Figure 1-7 A piston-cylinder devicearrow_forwardA variable-load piston-cylinder device contains air (cp = 1.005 kJ/kgK; cv = 0.718 kJ/kgK) at 500 kPa and T=12 oC. A paddle wheelequipped within the system and turned by an external electric motor until 65 kJ/kg of work has been transferred to the air. During this process the gas volume is quadrupled while maintaining the temperature constant by transferring heat to the gas. Determine (a) the final pressure, (b) the amount of required heattransfer (c) Show this process on a P-v diagram. Do not use Table A-17 while solving this problem. YOUR ANSWER SHEET SHOULD INCLUDE THE SOLUTION AND THE TABLE BELOW (a) Pinal [kPa] = (b) q [kJ/kg]arrow_forward
- Steam enters a nozzle at 400°C and 800 kPa with a velocity of 10 m/s and leaves at 375°C and 400 kPa while losing heat at a rate of 22.5 kW. For an inlet area of 800 cm2, determine the velocity and the volume flow rate of the steam at the nozzle exit. Use steam tables. 400°C 375°C 800 kPa Steam 400 kPa 10 m's The velocity of the steam at the nozzle exit is 2.06 m/s. The volume flow rate of the steam at the nozzle exit is 1.540 m3/s.arrow_forwardA piston-cylinder device contains 0.1 m³ of refrigerant 134a at 0.24 MPa and 40°C. Initially the piston is fixed with a pin. Heat is transferred now to the refrigerant from a source at 100°C until the pressure rises to 0.28 MPa. Then, heat is given to an environment with a temperature of 25°C at a constant pressure (the pin is pulled and in this case, the mass of the piston and the masses on it and the pressure created by the atmospheric pressure are equal to the pressure inside the cylinder) the temperature is brought to a temperature of 50°C. a) Determine the heat and work interaction for each process b) Sketch the P-v and T-s diagrams of the processes with respect to the saturation lines c) Determine the entropy change of the refrigerant 134a during these two processes d) Determine the entropy generation during these processes. Are these processes appropriate to 2. law of thermodynamics, explain.arrow_forwardA variable-load piston-cylinder device contains air (cp = 1.005 kJ/kgK; cv = 0.718 kJ/kgK) at 500 kPa and T=18 oC. A paddle wheel equipped within the system and turned by an external electric motor until 65 kJ/kg of work has been transferred to the air. During this process the gas volume is quadrupled while maintaining the temperature constant by transferring heat to the gas. Determine (a) the final pressure, (b) the amount of required heat transfer (c) Show this process on a P-v diagram. Do not use Table A-17 while solving this problemarrow_forward
- In a piston cylinder system, the gas an initial pressure of 100 kPa and expands from 1.5 m3 to 7.5 m3 with an increase in internal energy amounting to 160 kJ. Calculate the heat gain or loss in the system given the pressure volume relation as P2V is constant, where P is in kPa and V is in m3.arrow_forwardSteam enters the condenser of a steam power plant at 50 kPa and a quality of 85 percent with a mass flow rate of 400 kg/min. It is to be cooled by water from a nearby river by circulating the water through the tubes within the condenser. To prevent thermal pollution, the river water is not allowed to experience a temperature rise above 20°C. If the steam is to leave the condenser as saturated liquid at 50 kPa, determine the mass flow rate of the cooling water required.arrow_forwardConsider an evacuated rigid bottle of volume V that is surrounded by the atmosphere at pressure P0 and temperature T0. A valve at the neck of the bottle is now opened and the atmospheric air is allowed to flow into the bottle. The airtrapped in the bottle eventually reaches thermal equilibrium with the atmosphere as a result of heat transfer through the wall of the bottle. The valve remains open during the process so that the trapped air also reaches mechanical equilibrium with the atmosphere. Determine the net heat transfer through the wall of the bottle during this filling process in terms of the properties of the system and the surrounding atmosphere.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=NyOYW07-L5g;License: Standard youtube license