
Concept explainers
Interpretation:
The geometry of nitrogen atom and oxygen atom has to be predicted using VSEPR theory.
Concept Introduction:
Geometry of a compound is how the atoms that are present in the compound is connected in a 3D space. In order to find the geometry of the compound, the first thing that must be known was how the atoms bond with each other. Each and every atom in a compound is connected through bonds and the bonds are formed by overlapping orbitals.
Simple atomic orbitals are s and p orbitals. Generally in
To find the hybridization state of atom we start by counting the number of bonds the atom has and the lone pair of electrons present. The below table can be used to find the hybridization of atom,
| Sum of bonded atoms and lone pairs | Number of hybridized orbitals |
| 4 | 4 |
| 3 | 3 |
| 2 | 2 |
From the hybridization state of the atoms we can identify the geometry of the same. For finding the geometry, the lone pair of electrons also has to be considered. This complete details are given in the following table,
| Hybridization along with lone pair | Geometry |
| Tetrahedral | |
| Trigonal pyramidal | |
| Bent | |
| Trigonal planar | |
| Bent | |
| Linear |
Lone pair of electrons occupy the hybridized orbitals. If the same lone pair of electrons is involved in resonance means, then it does not occupy the hybridized orbital. Hence, the hybridization state of the atom changes which in turn changes the geometry of the atom.
Answer to Problem 4.18P
Nitrogen atom has trigonal planar geometry and oxygen atom has bent geometry.
Explanation of Solution
Given structure is,
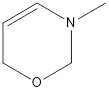
Redrawing the above structure with the lone pair of electrons,
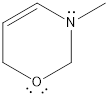
By looking at the structure given, we find that the nitrogen atom has three bonds and a lone pair of electrons. The nitrogen atom is considered to have
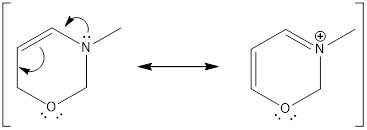
The oxygen atom has two bonds and two lone pair of electrons. Therefore, the hybridization of oxygen atom is
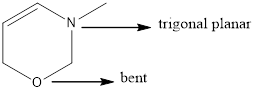
Geometry of the nitrogen and oxygen atom in the given structure was identified.
Want to see more full solutions like this?
Chapter 4 Solutions
ORGANIC CHEMISTRY-NEXTGEN+BOX (1 SEM.)
- Provide the correct common name for the compound shown here.arrow_forwardPh heat heatarrow_forward(12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





