
Concept explainers
(a)
Interpretation:
The most stable chair conformation of the given molecule is to be drawn in which a
Concept introduction:
According to VSEPR theory,
The most stable chair confirmation of disubstituted cyclohexane is the one in which the larger substituent occupies the equatorial position.
In disubstituted cyclohexane, the substituents which are on the same side are cis to each other whereas substituents which are on the opposite side of the ring are trans to each other. If there is more than one substituent attached, then the conformation in which maximum substituents are in equatorial position is favored and is the most stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip, whereas substituents that are cis remain cis to each other during the ring flip.
Answer to Problem 4.70P
The most stable conformation of the given molecule is:
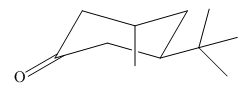
Explanation of Solution
The given molecule is:
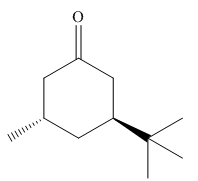
In the given molecule, there is a six member cyclic structure containing carbonyl group as a part of the ring. Thus, it is cyclohexanone. It has two substituents attached which are tertiary butyl group and a methyl group at C3 and C5 carbon atoms of cyclohexanone. Both substituents are trans to each other, as they lie on the opposite side of the ring. The tertiary butyl group is the bulkier substituent on the ring, and it is more stable in an equatorial position. Begin by drawing a chair conformation with a tertiary butyl group in an equatorial position. It is shown by a wedge bond, hence, it must point up in the chair conformation. The tertiary butyl group is pointed up and the methyl group must point down, for them to be trans, hence, the methyl group occupies the axial position as shown below:

If the chair is flipped, the equatorial tertiary butyl group becomes axial. The chair conformation having the bulkier tertiary butyl group in axial position is not stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has one substituent at equatorial position and another at axial position.
Interpretation:
The most stable chair conformation of the given molecule is to be drawn in which a
Concept introduction:
According to VSEPR theory,
The most stable chair confirmation of disubstituted cyclohexane is the one in which the larger substituent occupies equatorial position.
In disubstituted cyclohexane, the substituents which are on the same side are cis to each other whereas substituents which are on the opposite side of the ring are trans to each other. If there is more than one substituent attached, then the conformation in which maximum substituents are in equatorial position is favored and is the most stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip, whereas substituents that are cis remain cis to each other during the ring flip.
Answer to Problem 4.70P
The most stable conformation of the given molecule is:
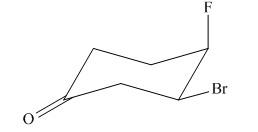
Explanation of Solution
The given molecule is:
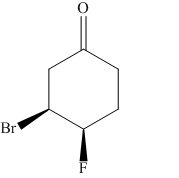
In the given molecule, there is a six member cyclic structure containing carbonyl group as a part of the ring. Thus, it is cyclohexanone. It has two substituents attached which are fluorine and bromine atoms at C3 and C4 carbon atoms of cyclohexanone. Both substituents are cis to each other, as they lie on the same side of the ring. Out of the two substituents, bromine atom is the largest substituent on the ring, and it is more stable in an equatorial position. Begin by drawing a chair conformation with bromine atom in equatorial position. It is shown by a wedge bond, hence, it must point up in the chair conformation. The bromine atom is pointed up and the fluorine atom must also point up for them to be cis, hence the fluorine atom occupies an axial position as shown below:

If the chair is flipped, the equatorial bromine atom becomes axial. The chair conformation having the bulkier group in equatorial position is more stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has one substituent at equatorial position and another at axial position.
(c)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
According to VSEPR theory,
The most stable chair confirmation of disubstituted cyclohexane is the one in which the larger substituent occupies the equatorial position.
In disubstituted cyclohexane, the substituents which are on the same side are cis to each other whereas substituents which are on the opposite side of the ring are trans to each other. If there is more than one substituent attached, then the conformation in which maximum substituents are in equatorial position is favored and is the most stable. Substituents that are trans to each other in one chair conformation remains trans after the chair flip, whereas substituents that are cis remain cis to each other during the ring flip.
Answer to Problem 4.70P
The most stable conformation of the given molecule is:
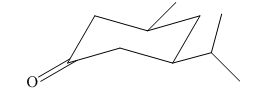
Explanation of Solution
The given molecule is:
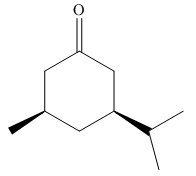
In the given molecule, there is a six member cyclic structure containing carbonyl group as a part of the ring. Thus, it is cyclohexanone. It has two substituents attached which are isopropyl group and methyl group at C3 and C5 carbon atoms of cyclohexanone. Both substituents are cis to each other as they lie on the same side of the ring. The isopropyl group is the largest substituent on the ring, and it is more stable in an equatorial position. Begin by drawing a chair conformation with isopropyl group in the equatorial position. It is shown by a wedge bond, hence, it must point up in the chair conformation. The isopropyl group is pointed up and the methyl group must also point up for them to be cis hence the methyl group goes to another equatorial position as shown below:

If the chair is flipped, both equatorial groups become axial. The chair conformation having the bulkier group in equatorial position is more stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has both substituents in equatorial position.
(d)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
According to VSEPR theory,
The most stable chair confirmation of disubstituted cyclohexane is the one in which the larger substituent occupies the equatorial position.
In disubstituted cyclohexane, the substituents which are on the same side are cis to each other whereas substituents which are on the opposite side of the ring are trans to each other. If there is more than one substituent attached, then the conformation in which maximum substituents are in equatorial position is favored and is the most stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip, whereas substituents that are cis remain cis to each other during the ring flip.
Answer to Problem 4.70P
The most stable conformation of the given molecule is:
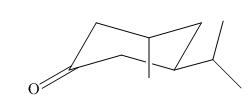
Explanation of Solution
The given molecule is:
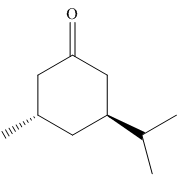
In the given molecule, there is a six member cyclic structure containing carbonyl group as a part of the ring. Thus, it is cyclohexanone. It has two substituents attached which are fluorine and bromine atoms at C3 and C4 carbon atoms of cyclohexanone. Both substituents are trans to each other, as they lie on the opposite side of the ring. Out of the two substituents, the isopropyl group is the largest substituent on the ring, and it is more stable in an equatorial position. Begin by drawing a chair conformation with isopropyl group in equatorial position. It is shown by a wedge bond, hence, it must point up in the chair conformation. The isopropyl group is pointed up and the methyl group must point down for them to be trans, hence the methyl group goes to axial position as shown below:

If the chair is flipped, the equatorial Isopropyl group becomes axial. The chair conformation having the bulkier group in equatorial position is more stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has one substituent at equatorial position and another at axial position.
(e)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
According to VSEPR theory,
The most stable chair confirmation of disubstituted cyclohexane is the one in which the larger substituent occupies the equatorial position.
In disubstituted cyclohexane, the substituents which are on the same side are cis to each other whereas substituents which are on the opposite side of the ring are trans to each other. If there is more than one substituent attached, then the conformation in which maximum substituents are in equatorial position is favored and is the most stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip, whereas substituents that are cis remain cis to each other during the ring flip.
Answer to Problem 4.70P
The most stable conformation of the given molecule is:
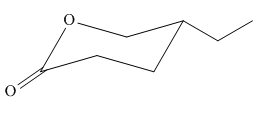
Explanation of Solution
The given molecule is:
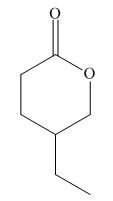
In the given molecule, there is a six membered cyclic structure containing ester group as a part of the ring. It has one substituent attached which is a ethyl group not shown by a wedge or dash bond. Thus, the ethyl group can be placed above or below the plane of the ring. The ethyl group is more stable at equatorial position than axial position. Thus, the most stable chair conformation of the given molecule is:

The most stable chair conformation of the given molecule has the bulkier substituent at equatorial position.
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry: Principles And Mechanisms
- Indicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are falsearrow_forward(f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward
- (c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- reaction scheme for C39H4202 Hydrogenation of Alkyne (Alkyne to Alkene) show reaction (drawing) pleasearrow_forwardGive detailed mechanism Solution with explanation needed. Don't give Ai generated solutionarrow_forwardShow work with explanation needed....don't give Ai generated solutionarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

