
Concept explainers
Fill in the blanks in each line of the following table. The first line is already completed as an example.
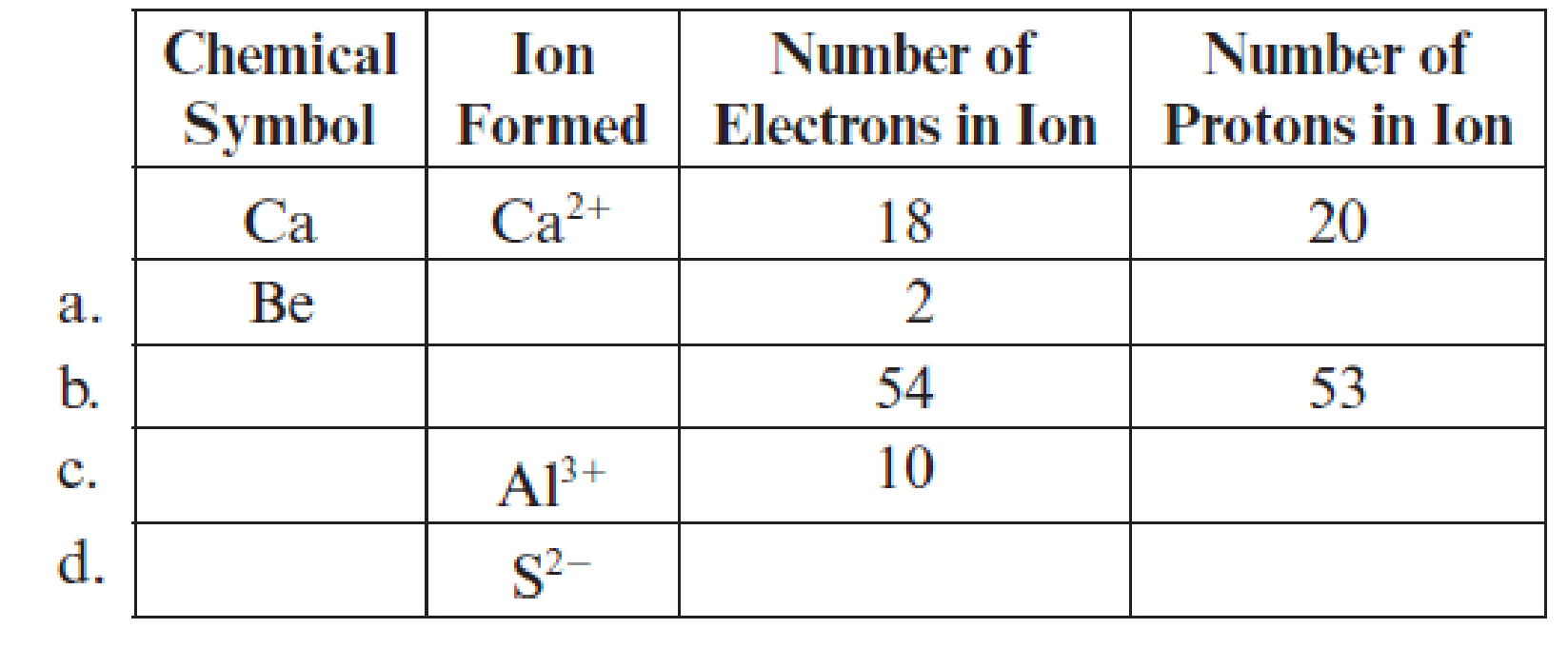
(a)
Interpretation:
Filling of each blank in the following table has to be done:
Concept Introduction:
Atoms are composed of three types of particles called subatomic particles. They are as follows:
- Protons: Positively charged particles in an atom.
- Neutrons: Neutral charged particles in an atom.
- Electrons: Negatively charged particles in an atom.
The neutral atom has equal number of protons and electrons. Gaining or loosing of electrons of an atom forms ion.
Negative charged ions are formed by gaining one or more electrons and it has more electrons than protons.
Positive charged ions are formed by losing one or more electrons and it has more protons than electrons.
Answer to Problem 4.29EP
Complete table is shown below:
Explanation of Solution
The chemical symbol of the element is
Two electrons are lesser than the total number protons thatmeans it lost two electrons and the charge of the beryllium ion is
Hence, the symbol of ion is
(b)
Interpretation:
Filling of each blank in the following table has to be done:
Concept Introduction:
Atoms are composed of three types of particles called subatomic particles. They are as follows:
- Protons: Positively charged particles in an atom.
- Neutrons: Neutral charged particles in an atom.
- Electrons: Negatively charged particles in an atom.
The neutral atom has equal number of protons and electrons. Gaining or loosing of electrons of an atom form ion.
Negative charged ions are formed by gaining one or more electrons and it has more electrons than protons.
Positive charged ions are formed by losing one or more electrons and it has more protons than electrons.
Answer to Problem 4.29EP
Complete table is shown below:
Explanation of Solution
The element has
Hence, the number of protons are
(c)
Interpretation:
Filling of each blank in the following table has to be done:
Concept Introduction:
Atoms are composed of three types of particles called subatomic particles. They are as follows:
- Protons: Positively charged particles in an atom.
- Neutrons: Neutral charged particles in an atom.
- Electrons: Negatively charged particles in an atom.
The neutral atom has equal number of protons and electrons. Gaining or loosing of electrons of an atom forms ion.
Negative charged ions are formed by gaining one or more electrons and it has more electrons than protons.
Positive charged ions are formed by losing one or more electrons and it has more protons than electrons.
Answer to Problem 4.29EP
Complete table is shown below:
Explanation of Solution
The ion
Hence, ion
(d)
Interpretation:
Filling of each blank in the following table has to be done:
Concept Introduction:
Atoms are composed of three types of particles called subatomic particles. They are as follows:
- Protons: Positively charged particles in an atom.
- Neutrons: Neutral charged particles in an atom.
- Electrons: Negatively charged particles in an atom.
The neutral atom has equal number of protons and electrons. Gaining or loosing of electrons of an atom forms ion.
Negative charged ions are formed by gaining one or more electrons and it has more electrons than protons.
Positive charged ions are formed by losing one or more electrons and it has more protons than electrons.
Answer to Problem 4.29EP
Complete table is shown below:
Explanation of Solution
The given ion is
The charge of sulfur atom is
Hence, ion is
Want to see more full solutions like this?
Chapter 4 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
 Medical Terminology for Health Professions, Spira...Health & NutritionISBN:9781305634350Author:Ann Ehrlich, Carol L. Schroeder, Laura Ehrlich, Katrina A. SchroederPublisher:Cengage Learning
Medical Terminology for Health Professions, Spira...Health & NutritionISBN:9781305634350Author:Ann Ehrlich, Carol L. Schroeder, Laura Ehrlich, Katrina A. SchroederPublisher:Cengage Learning

