
Concept explainers
Use a search engine such as Google to find information about Gordon E. Moore and Moore’s law, the famous law about technological advances that he proposed.
(a) What is Moore’s law? Give a brief description in your own words.
(b) Who is Gordon E. Moore? What was his position at the time he first proposed Moore’s law? What company did he later cofound? With whom did he cofound this company?
(c) In what field did Gordon E. Moore obtain his BS degree? At what university did he receive his BS degree? Where did he obtain his PhD degree? In what field was his PhD degree?
(d) What Nobel Prize-winning physicist gave Gordon E. Moore his first job opportunity?
(e) What was the number of the first microprocessor developed at Moore’s company and how many transistors did it have! When was it introduced!
(f) Read the article by Thomas Friedman on Moore’s law, and watch the video of his interview of Gordon E. Moore.9 In what year in what publication did Moore make his prediction? What was the most important lesson that Moore learned from his law? What stimulated his interest in science and engineering? What does Moore see as the biggest problem in science? How would you describe thestatus of Moore’s Law today!
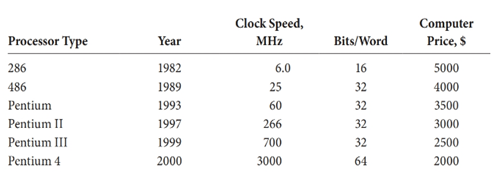
(g) One important benchmark ofcomputational progress is the performance-to-price ratio (PPR) of computers.10 The PPR is the number of bits per word divided by the product of cycle time (1/clockspeed) and price. The original IBM PC (1981) with an 8bit word length, a 4.77 MHz clock, and a pricetag of $5000 came in with a PPR of ~7600. Computers based on other processors available in 2000 are listed in the following table.10 Calculate the PPR of each of these computers. Does Moore’s law hold forthe PPR? How did you come to your conclusion?
Want to see the full answer?
Check out a sample textbook solution
- Mn, Tc, Re are elements of group VIIB and form oxoanions with a tetrahedral geometry. Is this statement correct?arrow_forwardGroup 7 elements (Mn, Tc, Re) form oxoanions with a tetrahedral geometry. Correct?arrow_forward. (8 pts.) Consider the stereochemical changes that accompany the dissociation mechanisms for ligand substitution reactions involving complexes of the form A-cis-[M(LL)2BX]. The three possible 5-coordinate intermediate ligand arrangements are shown. Draw all reaction pathways that lead to A product isomers. X B -B B - X -X B + Y - X B до + Y + Y ? ?arrow_forward
- If the boiling point of a K2SO4 solution is calculated to be 105.5, how much K2SO4 is dissolved in 150.0 g of water? Kb = 0.512 a. 280.8 g b. 93.60 g c. 4800. g d. 108.0 g e. 191.4 garrow_forwardDraw the product of the reaction shown below. Ignore inorganic byproducts. + O SnCl2 Drawingarrow_forwardQ4. Label the reaction most likely to take place (E1, SN1, E2, SN2 or a combination of these) under the following conditions. Draw the major product(s), include stereochemistry when relevant. a) b) tBuOK acetone CN CH3OHarrow_forward
- Show work with explanation needed. Don't give Ai generated solutionarrow_forward7 FREE RESPONSE SECTION - Show ALL work and write clearly. Circle or box your final answers to long problems! 16. (12 pts.) Name the following compounds. Be as descriptive as possible, using R/S and E/Z where needed. pricrity OH om 5 OH H H3C C-CC-CH3 OH Same sidearrow_forward1. Determine the amount of H2O2 when titrated with potassium permanganate solution 2 MnO4 (aq) + 5 H2O2(aq) + 5 H +(aq) 5 O2(g) + 2 Mn2+ (aq ) + 8 H2O(1)arrow_forward
- 85) Provide the major organic product of the reaction shown below. H OH HO 1. Ag₂O, CHI (excess) HO- H H OH 2. H₂Ot OCH3 Answer: Harrow_forwardProtonation reactions in metal clustersa) with multiple bonds take place on M-L bonds.b) take place on M-M bonds.c) take place on both types of bonds.arrow_forwardIndicate the correct answer.a) The H bridges in the B-H-B bonds behave as Bronsted acids.b) Boranes do not react with O2.c) None of them are correct.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





