
Concept explainers
(a)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(a)
Explanation of Solution
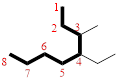
In the given compound, the longest carbon chain (highlighted with bold lines) contains EIGHT carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is OCTANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘4-ethyl-3-methyl-’
Therefore, the systematic name of the given compounds is ‘4-ethyl-3-methyloctane’.
(b)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(b)
Explanation of Solution
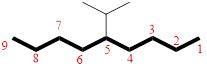
In the given compound, the longest carbon chain (highlighted with bold lines) contains NINE carbons and while numbering the parent chain, substituents should get the least possible number. The parent name is NONANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘5-isopropyl’
Therefore, the systematic name of the given compounds is ‘5-isopropylnonane’.
(c)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(c)
Explanation of Solution
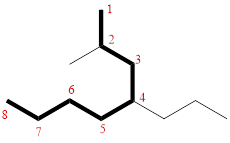
In the given compound, the longest carbon chain (highlighted with bold lines) contains EIGHT carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is OCTANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘2-methyl-4-propyl’
Therefore, the systematic name of the given compounds is ‘2-methyl-4-propyloctane’.
(d)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(d)
Explanation of Solution
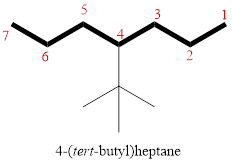
In the given compound, the longest carbon chain (highlighted with bold lines) contains SIX carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is HEXANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘4-(tert-butyl)’
Therefore, the systematic name of the given compounds is ‘4-(tert-butyl)heptane’.
(e)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(e)
Explanation of Solution
In the given compound, the longest carbon chain (highlighted with bold lines) contains ELEVEN carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is UNDECANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘5-(sec-butyl)-4-ethyl-2-methyl’
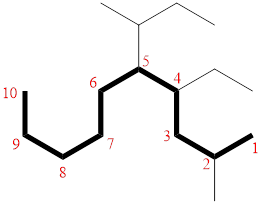
Therefore, the systematic name of the given compounds is ‘5-(sec-butyl)-4-ethyl-2-methyldecane’.
(f)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(f)
Explanation of Solution
In the given compound, the longest carbon chain (highlighted with bold lines) contains TEN carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is DECANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘3-ethyl-6-isopropyl-2,4-dimethyl’
Therefore, the systematic name of the given compounds is ‘3-ethyl-6-isopropyl-2,4-dimethyl decane’.
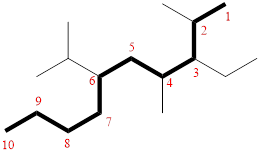
(g)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(g)
Explanation of Solution
In the given compound, the longest carbon chain (highlighted with bold lines) contains EIGHT carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is OCTANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘3,5-diethyl-2-methyl’
Therefore, the systematic name of the given compounds is ‘3,5-diethyl-2-methyloctane’.
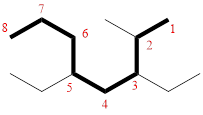
(h)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(h)
Explanation of Solution
In the given compound, the longest carbon chain (highlighted with bold lines) contains SEVEN carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is HEPTANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘2,3,5-trimethyl-4-propyl’
Therefore, the systematic name of the given compounds is ‘2,3,5-trimethyl-4-propylheptane’.
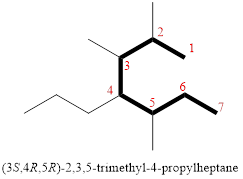
(i)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(i)
Explanation of Solution
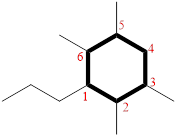
In the given compound, the number of carbon involving in ring formation is called parent ring
(Highlighted with bold lines) contains SIX carbons and while numbering the parent ring, substituents should get the least possible number. The parent name is CYCLOHEXANE. The substituents are arranged in alphabetical order followed by the parent name.
Then as usual, the substituents are arranged in alphabetical order as ‘2,3,5,6-tetramethyl-1-propyl-’
Therefore, the systematic name of the given compounds is ‘2,3,5,6-tetramethyl-1-propyl cyclohexane’.
(j)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Bicyclic compounds: Compounds that contain two fused rings called bicyclic compounds.
Nomenclature of bicyclic compounds:
The organic compound naming is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Identify and the parent: The term ‘Bicylo-’ is introduced in the name of the parent. Count the number of carbons excluding the bridge heads. In the compound below, each of the three paths has two carbons. These three numbers are ordered from largest to smallest, as [2.2.2] and placed in the middle of the parent
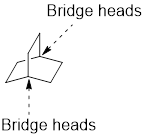
Identify and name substituents: If substituent is present, the parent must be numbered properly in order to assign the locants to the substituent. To number the parent, start at one of the bridgeheads and begin numbering along the longest path, then go to the second longest path, and finally go along the shortest path.
Arrange the substituents alphabetically.
In the complex substituent in compounds, the substituent name is assigned by a name each of them based on numbers going away from the parent.
(j)
Explanation of Solution
The cyclic system has ten carbons and the parent name is DECANE. The term ‘Bicylo-’ is introduced in the name of the parent. On Counting the number of carbons excluding the bridge heads three numbers like (4, 4, and 0) are ordered from largest to smallest, as [4.4.0] and placed in the middle of the parent as bicyclo[4.4.0]decane.
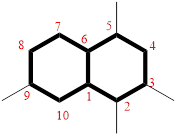
Then as usual, the substituents are arranged in alphabetical order as ‘2,3,5,9-tetramethyl’
Therefore, the systematic name of the given hydrocarbon core is 2,3,5,9-tetramethylbicyclo[4.4.0]decane.
(k)
Interpretation:
The systematic name of given compounds has to be provided.
Concept Introduction:
Bicyclic compounds: Compounds that contain two fused rings called bicyclic compounds.
Nomenclature of bicyclic compounds:
The organic compound naming is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Identify and the parent: The term ‘Bicylo-’ is introduced in the name of the parent. Count the number of carbons excluding the bridge heads. In the compound below, each of the three paths has two carbons. These three numbers are ordered from largest to smallest, as [2.2.2] and placed in the middle of the parent

Identify and name substituents: If substituent is present, the parent must be numbered properly in order to assign the locants to the substituent. To number the parent, start at one of the bridgeheads and begin numbering along the longest path, then go to the second longest path, and finally go along the shortest path.
Arrange the substituents alphabetically.
In the complex substituent in compounds, the substituent name is assigned by a name each of them based on numbers going away from the parent.
(k)
Explanation of Solution
The cyclic system has ten carbons and the parent name is OCTANE. The term ‘Bicylo-’ is introduced in the name of the parent. On Counting the number of carbons excluding the bridge heads three numbers like (2, 2, and 2) are ordered from largest to smallest, as [2.2.2] and placed in the middle of the parent as bicyclo[2.2.2]octane.
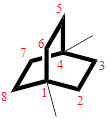
Then as usual, the substituents are arranged in alphabetical order as ‘1,4-dimethyl’
Therefore, the systematic name of the given hydrocarbon core is 1,4-dimethylbicyclo[2.2.2]octane.
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





