
Concept explainers
The Madelung constant (Section 37.3) is notoriously difficult to calculate because it’s the sum of an alternating series of nearly equal terms. But it can be calculated for a hypothetical one-dimensional crystal consisting of an evenly spaced line of alternating positive and negative ions (Fig. 37.25). Show that the potential energy of an ion in this “crystal” can be written as
where the Madelung constant α has the value 2 ln2.
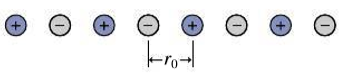
FIGURE 37.25 Problem 61
Want to see the full answer?
Check out a sample textbook solution
Chapter 37 Solutions
Essential University Physics (3rd Edition)
Additional Science Textbook Solutions
Introduction to Electrodynamics
The Cosmic Perspective Fundamentals (2nd Edition)
Mathematical Methods in the Physical Sciences
Fundamentals of Physics Extended
Fundamentals Of Physics - Volume 1 Only
The Cosmic Perspective (8th Edition)
- At what temperature, in terms of Tc, is the critical field of a superconductor one-half its value at T = 0 K?arrow_forwardIn a Si semiconductor sample of 200 am length at 600 K the hole concentration as a' function of the sample length follows a quadratic relation of the form p (x) = 1 x1015x, at equilibrium the value of the electric field at 160 jum will be: O 1.935 V/cm O 3.250 V/cm O 5805 V/cm O 55.56 V/cm O 6.450 V/cmarrow_forwardAn n-type defect in a semiconductor acts like a hydrogenic atom, with the exception that the dielectric constant of the vacuum is replaced by the dielectric constant for Si. If the dielectric constant for Si is 11.7 times larger than for the vacuum. The ionization energy for an electron in an n-type defect is approximately: А. 3.4 ev В. 0.1 ev С. 13.6 ev D. 1.2 evarrow_forward
- d) Show that the resistivity canbe expressed (via the familiar notation) as, m p= net here m is the electronmass; † is the period of time that elapses between two subsequent collisions, the electron undergoes with copper nuclei throughout its chaotic motion in the body of copper (supposing that the current carrying cable is made of copper). Hint: Write F=eE, i.e. the electric force reigning on the electron. Write also the acceleration a=F/m, an electron is subject to, based on the Newton's law ofmotion.arrow_forwardThe potential energy of a system of two atoms is given by the relation U =-A/r + B/r10 A stable molecule is formed with the release of 8 eV energy when the interatomic distance is 2.8 Å. Find A and B and the force needed to dissociate this molecule into atoms and the interatomic distance at which the dissociation occurs.arrow_forwardTo obtain the value of an unknown electrical charge, a group performed an experiment. From the graph of the electric potential (voltage) V in volts, as a function of the inverse distance (1/r) in m^-1, the group obtained an angular coefficient 6838 Nm^2/C by the linear equation of the best straight line. Knowing that V=(kq)/r, calculate the value of the electric charge, in nC (nanocoulomb), from the slope provided by the best line. Round the answer to a whole number. Use: k = 8.9876 x 10^9 N⋅m^2⋅C^−2arrow_forward
- Graph below shows the electron occupancy probability P(E) as a function of energy for Bismuth (mBi = 3.47 × 10-25 kg) at the temperature T = 0 K. What is the number of conduction electrons per unit volume for Bismuth? 1 1 2 3 4 5 6 7 8 E (ev) P(E)arrow_forwardIn solid KCI the smallest distance between the centers of a. potassium ion and a chloride ion is 314 pm. Calculate the length of the edge of the unit cell and the density of KCI, assuming it has the same structure as sodium chloride.arrow_forwardOne model for the potential energy of a two-atom molecule, where the atoms are separated by a distance r, is U(r) = Uo[(¹) ¹2 – ( )²] where ro = 0.8 nm and U₁ = 6.1 eV. Note: 1 eV = 1.6 × 10-19 J. Some helpful units: [Force] = eV/nm [Energy] = eV [distance] = nm Equilibrium Distance What is the distance between the atoms when the molecule is in stable equilibrium? Click here for a hint T'eq Hint: Hint: Hint: Hint: Hint: Hint: Force If the distance between the atoms increases from equilibrium by r₁ = 0.35 nm, then what is the force from one atom on the other associated with this potential energy? (Enter your answer as postive if they repel each other, and negative if they attract.) Fr(req+r₁) Hint: Hint: 0.89105934nm Kinetic Energy Hint: The atoms are oscillating back and forth. The maximum separation of the atoms is r₂ = 2 nm. What is the kinetic energy of the atoms when they are separated by the equilibrium distance? Click here for a hint K(req) Hint: Hint: = -1.288eV/nm 3.99eVarrow_forward
- X- Hall Effect demonstrates that it is the electrons that are free to move. Y- Germanium and Selenium are materials that are intermediate between insulators and conductors. O X is true and Y is false O X is false and Y is true O X and Y are both true O X and Y are both falsearrow_forwardA brittle material has the properties Sut=30 kpsi and Suc=90 kpsi. Using the brittle Coulomb- Mohr and modified-Mohr theories, determine the factor of safety for the following states of plane stress. Ox=-15 kpsi, o,=10 kpsi, txy=-15 kpsiarrow_forwardThe gap between valence and conduction bands in diamond is 5.47 eV. (a) What is the maximum wavelength of a photon that can excite an electron from the top of the valence band into the conduction band? In what region of the electromagnetic spectrum does this photon lie? (b) Explain why pure diamond is transparent and colorless. (c) Most gem diamonds have a yellow color. Explain how impurities in the diamond can cause this color.arrow_forward
 University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
