
Concept explainers
Interpretation : We need to interpret the formation of benzene as the side product during the Grignard reaction while using phenyl magnesium bromide with the help of a balanced chemical equation.
Concept Introduction : Grignard reagent is a chemical compound with a general formula
The Grignard reagent is widely used in the
Answer to Problem 1Q
The hydrolysis of phenyl magnesium bromide forms benzene as the side product. The balanced chemical equation can be written as:
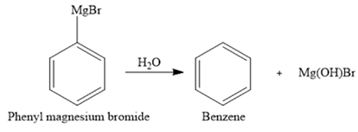
Explanation of Solution
In the Grignard reagent, the alkyl or aryl group acts as a strong nucleophile therefore it can react easily with carbonyl compounds to form alcohols. It can readily react with water that breaks the
Thus, hydrolysis of phenyl magnesium bromide forms benzene as a side product. This can occur if the glassware is not completely dry. The balanced chemical equation can be written as:
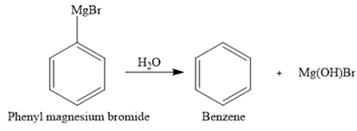
Grignard reagent is unstable in water and readily decomposes to alkane and respective alcohol.
Want to see more full solutions like this?
Chapter 33 Solutions
EBK A SMALL SCALE APPROACH TO ORGANIC L
- FeCl3 or AlCl3 are also generally used as catalysts for Friedel-Crafts alkylations. Why might one start with Al as the catalyst starting point instead?arrow_forwardWhat is the objective for preparation of p-Iodonitrobenzene?arrow_forwardWhat color will be produced if cyclohexene and toluene are reacted with sulfuric acid?arrow_forward
- TiO2 is one of the most useful materials to decompose organic substances using photocatalytic oxidation. What final products of photocatalytic oxidation over TiO2 do you expect for the following two substances: phenyl isothiocyanate and trinitrotoluene, assuming complete mineralization of these compounds in photocatalytic processes? Write a chemical formula for a whole reaction for each compound and explain the reactions.arrow_forwardWhat is the reaction equation for preparation of p-Iodonitrobenzene?arrow_forwardAssuming the inorganic product of Na2S2O4. 2H2O is HSO3-, write a balanced equation for the reaction of 3-nitrophthalhydrized with Na2S2O4. 2H2O.arrow_forward
- Hydrocarbon S, C8H8 reacts with hydrogen in the presence of platinum catalyst to yield T, C8H10. Compound U is formed when T is heated with alkaline potassium permanganate solution, followed by hydrolysis. The reaction of S with hydrogen bromide V, whereas ozonolysis of S produces W and methanal. Draw the structures of S to W. Give the name of the reaction for the conversion of S to T. Write all chemical equations involved.arrow_forwardWhat organometallic compound is formed from the reaction of excess methylmagnesium chloride and GaCl3?arrow_forwardWhy cis-Ru(II)Cl2(DMSO)4 reacts with pyridine, et cetera, to give substitution of the DMSO but not the chloride ligands, but trans-Ru(II)Cl2(py)4 react with suitable Na+ and K+ salts in aqueous pyridine to afford chloride-substituted derivatives. write the reactions equations.arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

