
One component of jojoba oil is a wax formed from eicosenoic acid
Interpretation: The structure of the wax formed from eicosenoic acid
Concept introduction: Waxes are lipids, which are hydrolysable. They contain ester
Answer to Problem 31.1P
The structure of the wax formed from eicosenoic acid
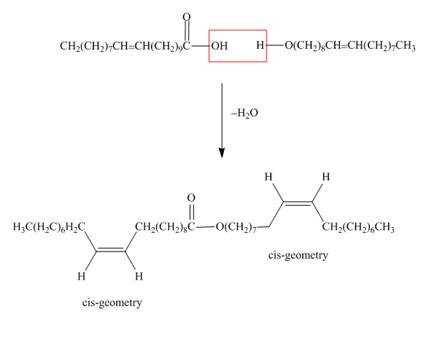
Figure 1
Explanation of Solution
Waxes are lipids, which are hydrolysable. They contain ester
The treatment of eicosenoic acid
The structure of the wax formed from eicosenoic acid
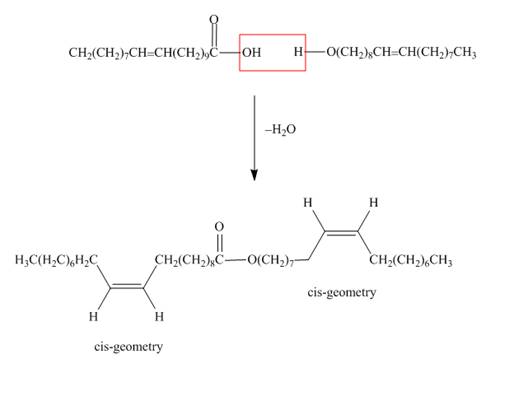
Figure 1
The structure of the wax formed from eicosenoic acid
Want to see more full solutions like this?
Chapter 31 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Name these organic compounds: structure name CH, CH;- C- CH3 CH2 CH, — CH, — с — CH; | CH; CH,- CH –CE CHarrow_forwardName these organic compounds: structure name CH, CH, — сн, — с — сн, CH2 CH, — с — сн, CH; CH;- CH – C= CHarrow_forwardIs the compound CH3CH=CH2 saturated or unsaturated? Explain.arrow_forward
- Rank the following compounds in order of increasing water solubility: glucose, hexane [CH 3(CH 2) 4CH 3], and 1-decanol [CH 3(CH 2) 9OH]. Explain your choice.arrow_forwardGasohol is a mixture of 90% gasoline and 10% ethanol, CH 3CH 2OH. Ethanol is considered an environmentally friendly fuel additive because it can be made from a renewable source—sugarcane. Ethanol burns in air to form CO 2 and H 2O, and, like the combustion of alkanes, this reaction also releases a great deal of energy. Write a balanced equation for the combustion of ethanol.arrow_forwardGlucose, C6H12O6, contains an aldehyde group but exist predominantly in the form of the cyclic hemiacetal show below. A cyclic hemiacetal is formed when the —OH group of one carbon bonds to the carbonyl group of another carbon. Identify which carbon provides the —OH group and which provides the —CHO? Give a functional isomer of glucose and draw its structure.arrow_forward
- Consider the following reactions: When C5H12 is reacted with Cl2(g) in the presence of ultraviolet light, four different monochlorination products form. What is the structure of C5H12 in this reaction? When C4H8 is reacted with H2O, a tertiary alcohol is produced as the major product. What is the structure of C4H8 in this reaction? When C7H12 is reacted with HCl, 1-chloro-1-methylcyclohexane is produced as the major product. What are the two possible structures for C7H12 in this reaction? When a hydrocarbon is reacted with water and the major product of this reaction is then oxidized, acetone (2-propanone) is produced. What is the structure of the hydrocarbon in this reaction? When C5H12O is oxidized, a carboxylic acid is produced. What are the possible structures for C5H12O in this reaction?arrow_forward4. Write the structure of the product resulting from the reaction of CH3 H,C- CH2arrow_forwardDraw a chiral alkene with the formula C6H12.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning



