
Prescott's Microbiology
10th Edition
ISBN: 9781259281594
Author: Joanne Willey, Linda Sherwood Adjunt Professor Lecturer, Christopher J. Woolverton Professor
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 30.1, Problem 1MI
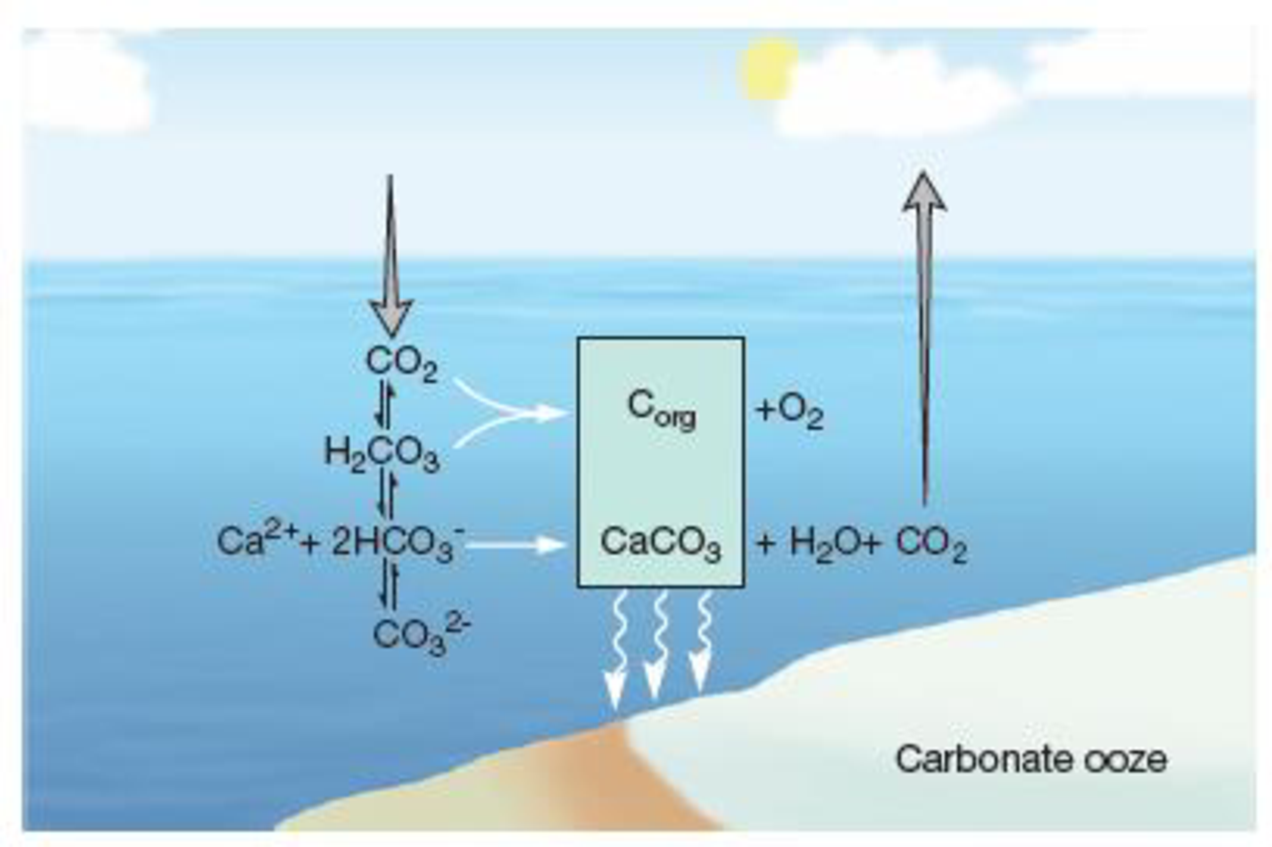
Figure 30.1 The Carbonate Equilibrium System. Atmospheric CO2 enters seawater and is converted to organic carbon (Corg) or to carbonic acid (H2CO3) that rapidly dissociates into the weak acids bicarbonate (HCO3−) and carbonate (CO32−). Calcium carbonate (CaCO3), a solid, precipitates to the seafloor, where it helps form a carbonate ooze. This system keeps seawater buffered at about pH 8.0.
Which carbon species, CO2 or CO32−, acidifies seawater?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Water circulation is complex.Arrange five distinctive steps of water that continually cycle around the planet.
Which of has following group of gases contribute to the 'Green House Effect' ?
Carbon tetrafluoride and Nitrous oxide
Carbon monoxide and Sulphur dioxide
Ammonia and Ozone
Carbon dioxide and Methane
"If you add carbon dioxide to seawater, as occurs when bacteria or animals respire or atmospheric CO2 dissolves into seawater, which of the following will happen?"
Some of the H+ ions in seawater will combine with CO2 to make HCO2
The carbon dioxide will combine with oxygen and reduce the oxygen concentration.
"The H+ derived from CO2 will combine with carbonate, CO32-, to form bicarbonate"
pH will go up.
"Nothing will happen, since the ocean water is well buffered via its alkalinity. "
Chapter 30 Solutions
Prescott's Microbiology
Ch. 30.1 - Figure 30.1 The Carbonate Equilibrium System....Ch. 30.1 - What factors influence oxygen solubility? How is...Ch. 30.1 - Describe the buffering system that regulates the...Ch. 30.1 - Prob. 3RIACh. 30.1 - What features of a thermocline make it similar to...Ch. 30.2 - How is sulfur cycled between the anoxygenic...Ch. 30.2 - How do heterotrophic microbes contribute to the...Ch. 30.2 - Prob. 3MICh. 30.2 - Prob. 1.1RIACh. 30.2 - Prob. 1.2RIA
Ch. 30.2 - Describe the ecosystem that develops within a...Ch. 30.2 - What is marine snow? Why is it important in CO2...Ch. 30.2 - Prob. 2.2RIACh. 30.2 - Why do you think that, despite their great...Ch. 30.2 - List some metabolic strategies that have evolved...Ch. 30.2 - Describe the role of marine viruses in the...Ch. 30.2 - Explain what is meant by upside-down microbial...Ch. 30.2 - Prob. 2.7RIACh. 30.3 - Figure 30.15 Nutrient Cycling in Antarctic Lakes...Ch. 30.3 - How does the contribution of benthic autotrophs...Ch. 30.3 - Why does water turbulence play only a minor role...Ch. 30.3 - Why is mixotrophy suited for survival in Antarctic...Ch. 30.3 - What is an oxygen sag curve? What changes in a...Ch. 30.3 - What are point and nonpoint source pollution? Can...Ch. 30.3 - Prob. 2.1RIACh. 30.3 - Prob. 2.2RIACh. 30.3 - Why do cyanobacteria often dominate waters that...Ch. 30 - The unicellular cyanobacterium Prochlorococcus sp....Ch. 30 - How might it be possible to cleanse an aging...Ch. 30 - It is well known that bacterivory (the consumption...Ch. 30 - Prob. 4CHI
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- In the lungs, the main factor in boosting the tendency of hemoglobin to bind with and hold oxygen is ___. a. temperature c. acidity (pH) b. the amount of O2 relative to the amount of CO2 in plasma c. acidity(pH) d. all are equally importantarrow_forwardarrow_forwardA water molecule has polar O-H bonds that result in regions of partial positive charge (hydrogen atoms) and a region of partial negative charge (oxygen atom with lone pairs). Place the Na+ and CI ions where H₂O molecules are properly oriented to form ion-dipole interactions. Answer Bank CI Natarrow_forward
- As carbon dioxide levels rise in atmosphere, it is absorbed by oceans and the acidity of oceans increases damaging marine life. True Falsearrow_forward. An analysis water from well No 9 of the Manhattan beach, California, water composition showed : pH=7.8; silica: 19.2 mg SiO2/liter; calcium = 60 mg Ca2+/liter ; magnesium = 29.16 mg Mg2+/liter; sodium=78.2 mg Na+ /liter; potassium : 42.9 mg K+ /liter; bicarbonate= 292.8 mg HCO3 - /liter; sulfate=134.82 mg S04 2- /liter; chloride = 99.4 mg Cl- /liter and nitrate =6.2 mg NO3 - /liter. The relative atomic masses are: Si=28; Ca=40; Mg=24.3; Na= 23; K=39; O=16; H=1; C=12; S=32; Cl=35.5; N=14. (a) Calculate the ionic balance (b) Is the water analysis satisfactory from the point of view of analytical accuracy? Justify your answerarrow_forwardWhich one of the following statements is false? Group of answer choices Water plays the main role in the increase in the mean global temperature referred to as global warming. Water does not cause global warming because the atmospheric lifetime of water vapor is short. The amount of energy absorbed when water evaporates is large due to its strong intermolecular forces. Water absorbs the greatest percentage of infrared radiation being emitted by the earth. The energy when released water condenses heats the atmosphere and fuels storms.arrow_forward
- Father's Genes Hemophilia is a sex-linked, recessive trait. Which of the following describes the probability of hemophilia in the offspring of a man who does not have hemophilia and a woman who is a heterozygous carrier? You may use a Punnett Square like the one below to find your answer There is a 0% chance that their daughters will have hemophilia O There is a 50% chance that their daughters will have hemophilia There is a 25% chance that their sons will have hemophilia There is a 100% chance that their sons will have hemophilia O o oarrow_forwardVolcanoes emit much hywdrogen sulfide gas, H2S, which reacts with the oxygen in the air to form water and sulfur dioxide, SO2. Every 83 tons of H2S reacts with 117 tons of oxygen and forms 44 tons of water. How many tons SO2 are formed.arrow_forwardfill in the bubblesarrow_forward
- Which of the following statements accurately describes water vapor? The liquid phase of water is water vapor Water vapor is the water's solid phase. Water vapor is water in its gaseous state All of the above The zone composed of air, water, and soil is referred to as Atmosphere Biosphere Hydrosphere Lithosphere 3.Do greenhouse gases serve any purpose on Earth? No, they merely block a portion of the Sun's rays. They do, in fact, keep the Earth warm. Yes, they do maintain all of the gases on our planet in place. No, they merely contribute to catastrophic climate change.arrow_forwardACID RAIN Acid rain occurs due to the presence of certain pollutants in the atmosphere. Acid raiñ"can belcaused by the combustion of fossil tuels, erupting volcanges, or rotting vegetation that releases sulfur dioxide and nitrogen oxide into the atmosphere. When fossil fuels such as coal, gasoline, and fuel oils are burned, they emit oxides of sulfur, carbon, and nitrogen into the air. These oxides combine with moisture in the air to form sulfuric acid, carbonic acid, and nitric acid. The term "acid rain" is also applied to other forms of precipitation-snow, hail, sleet, and fog-that are similarly acidic. During the 20th century, acid rain was recognized as a leading threat to the Earth's environment. Most acid rain comes from fossil fuel emissions produced in the industrialized northern hemisphere--the United States, Canada, Asia, and most of Europe. Acid rain is devastating to all forms of life, but its effects are especially severe in freshwater habitats such as lakes, rivers, and…arrow_forwardIn the supermarket why do they their produce with water on a regular basis? this should be an original thought, not Google’s. 3-4 sentencesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
 Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning


Concepts of Biology
Biology
ISBN:9781938168116
Author:Samantha Fowler, Rebecca Roush, James Wise
Publisher:OpenStax College


Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning

Microorganisms | Genetics | Biology | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=YSitT0oOoyc;License: Standard youtube license