
University Physics Volume 2
18th Edition
ISBN: 9781938168161
Author: OpenStax
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3, Problem 58P
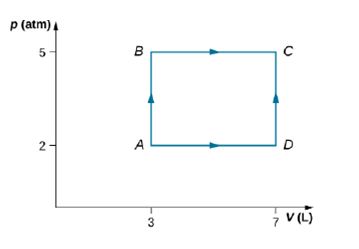
Consider the processes shown below. In the processes AB and BC, 3600 J and 2400 J of heat are added to the system, respectively. (a) Find the work done in each of the processes AB, BC, AD, and DC. (b) Find the internal energy change in processes AB and BC. (c) Find the internal energy difference between states C and A. (d) Find the total heat added in the ADC process. (e) From the information give, can you find the heat added in process AD? Why or why not?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A heat pump is used to keep a house warm at 22∘C.
How much work is required of the pump to deliver 2700 JJ of heat into the house if the outdoor temperature is 0∘C∘C. Assume a COP of 3.0
Express your answer to two significant figures and include the appropriate units.
How much work is required of the pump to deliver 2700 JJ of heat into the house if the outdoor temperature is -15∘C∘C? Assume a COP of 3.0
Express your answer to two significant figures and include the appropriate units.
How much work is required of the pump to deliver 2700 JJ of heat into the house if the outdoor temperature is 0∘C∘C. Assume an ideal (Carnot) coefficient of performance COPCOP = TH/(TH−TL)TH/(TH−TL).
Express your answer to two significant figures and include the appropriate units
How much work is required of the pump to deliver 2700 JJ of heat into the house if the outdoor temperature is -15∘C∘C. Assume an ideal (Carnot) coefficient of performance COPCOP = TH/(TH−TL)TH/(TH−TL).
Express your…
During a thermodynamic process, a system moves from state A to state B. It is supplied with
500 J of heat and does 300 J of work. (a) For this transition, what is the change in the system's
total internal energy? (b) If the system then moves from state B back to state A, what is the
change in its total internal energy from B to A? (c) If in moving from A to B along a different
400 J of work is done on the system, how much heat does it absorb? Remember
the change in internal energy is independent of the path.
path, WAB
=
A coal-fired power station is a huge heat engine. It uses heat transfer from burning coal to do work to turn turbines, which are used to generate electricity. In a single day, a large coal power station has 2.50×1014 J of heat transfer from coal and 1.48×1014 J of heat transfer into the environment. (a) What is the work done by the power station? (b) What is the efficiency of the power station?(c) In the combustion process, the following chemical reaction occurs: C + O2 → CO2 .This implies that every 12 kg of coal puts 12 kg + 16 kg + 16 kg = 44 kg of carbon dioxide into the atmosphere. Assuming that 1 kg of coal can provide 2.5×106 J of heat transfer upon combustion, how much CO2 is emitted per day by this power plant?
Chapter 3 Solutions
University Physics Volume 2
Ch. 3 - The paths ABC, AC, and ADC represent three...Ch. 3 - Check Your Understanding The quantities below...Ch. 3 - Check Your Understanding Why was it necessary to...Ch. 3 - Check Your Understanding When 1.00 g of ammonia...Ch. 3 - Consider these scenarios and state whether work is...Ch. 3 - Is it possible to determine whether a change in...Ch. 3 - When a liquid is vaporized, its change in internal...Ch. 3 - Why does a bicycle pump feel warm as you inflate...Ch. 3 - Is it possible for the temperature of a system to...Ch. 3 - What does the first law of thermodynamics tell us...
Ch. 3 - Does adding heat to a system always increase its...Ch. 3 - A great deal of effort, time, and money has been...Ch. 3 - When a gas expands isothermally, it does work....Ch. 3 - If the pressure and volume of a system are given,...Ch. 3 - It is unlikely that a process can be isothermal...Ch. 3 - How can an object transfer heat if the object does...Ch. 3 - Most materials expand when heated. One notable...Ch. 3 - Why are there two specific heats for gases Cp and...Ch. 3 - Is it possible for to be smaller than unity? `Ch. 3 - Would you expect to be larger for a gas or a...Ch. 3 - There is no change in the internal of an ideal gas...Ch. 3 - Does a gas do any work when it expands...Ch. 3 - A gas follows on an isothermal curve, where p is...Ch. 3 - A mole of gas has isobaric expansion coefficient...Ch. 3 - Find the equation of state of a solid that has an...Ch. 3 - A gas at a pressure of 2.00 atm undergoes a...Ch. 3 - It takes 500 J of work to compress...Ch. 3 - It is found that, when a dilute gas expands...Ch. 3 - In a quasi-static isobaric expansion. 500 J of...Ch. 3 - When a gas undergoes a quasi-static isobaric...Ch. 3 - An ideal gas expands quasi-statically and...Ch. 3 - As shown below, calculate the work done by the gas...Ch. 3 - (a) Calculate the work done by the gas along the...Ch. 3 - An ideal gas expands quasi-statically to three...Ch. 3 - A dilute gas at a pressure of 2.0 atm and a volume...Ch. 3 - What is the average mechanical energy of the atoms...Ch. 3 - What is the internal energy of 6.00 mol of an...Ch. 3 - Calculate the internal energy of 15 mg of helium...Ch. 3 - Two monatomic ideal gases A and B are at the same...Ch. 3 - The van der Waals coefficients for oxygen are...Ch. 3 - Find the work done in the quasi-static processes...Ch. 3 - When a dilute gas expands quasi-statically from...Ch. 3 - In a quasi-static isobaric expansion, 500 J of...Ch. 3 - An ideal gas quasi-statically and isothermally...Ch. 3 - As shown below, if the heat absorbed by the gas...Ch. 3 - During the isobaric expansion from A to B...Ch. 3 - (a) What is the change in internal energy for the...Ch. 3 - When a gas expands along path AC shown below, it...Ch. 3 - When a gas expands along AB (see below), it does...Ch. 3 - A dilute gas is stored in the left chamber of a...Ch. 3 - Ideal gases A and B are stored in the left and...Ch. 3 - An ideal monatomic gas at a pressure of 2.0105N/m2...Ch. 3 - Consider the process for steam in a cylinder shown...Ch. 3 - The state of 30 moles of steam in a cylinder is...Ch. 3 - A monatomic ideal gas undergoes a quasi-static...Ch. 3 - A metallic container of fixed volume of 2.5103 m3...Ch. 3 - A gas in a cylindrical closed container is...Ch. 3 - Two moles of a monatomic ideal gas at (5 MPa, 5 L)...Ch. 3 - Consider a transformation from point A to B in a...Ch. 3 - Consider a cylinder with a movable piston...Ch. 3 - An ideal gas expands isothermally along AB and...Ch. 3 - Consider the processes shown below. In the...Ch. 3 - Two moles of helium gas axe placed in a...Ch. 3 - An amount of n moles of a monatomic ideal gas in a...Ch. 3 - The temperature of an ideal monatomic gas rises by...Ch. 3 - For a temperature increase of 10 at constant...Ch. 3 - If the gases of the preceding problem are...Ch. 3 - Consider 0.40 mol of dilute carbon dioxide at a...Ch. 3 - When 400 J of heat are slowly added to 10 mol of...Ch. 3 - One of a dilute diatomic gas occupying a volume of...Ch. 3 - A monatomic ideal gas undergoes a quasi-static...Ch. 3 - An ideal gas has a pressure of 0.50 atm and a...Ch. 3 - Pressure and volume measurements of a dilute gas...Ch. 3 - An ideal monatomic gas at 300 K expands...Ch. 3 - An ideal diatomic gas at 80 K is slowly compressed...Ch. 3 - An ideal diatomic gas at 80 K is slowly compressed...Ch. 3 - Compare the charge in internal energy of an ideal...Ch. 3 - The temperature of n moles of an ideal gas changes...Ch. 3 - A dilute gas expands quasi-statically to three...Ch. 3 - (a) An ideal gas expands adiabatically from a...Ch. 3 - On an adiabatic process of an ideal gas pressure,...Ch. 3 - Two moles of a monatomic ideal gas such as helium...Ch. 3 - Consider the process shown below. During steps AB...Ch. 3 - A car tile contains 0.0380 m3 of air at a pressure...Ch. 3 - A helium-filled toy balloon has a gauge pressure...Ch. 3 - Steam to drive an old-fashioned steam locomotive...Ch. 3 - A hand-driven tire pump has a piston with a...Ch. 3 - Calculate the net work output of a heat engine...Ch. 3 - What is the net work output of a heat engine that...Ch. 3 - Five moles of a monatomic ideal gas in a cylinder...Ch. 3 - Four moles of a monatomic ideal gas in a cylinder...Ch. 3 - Helium gas is cooled from 20 to 10 by expanding...Ch. 3 - In an adiabatic process, oxygen gas in a container...Ch. 3 - A cylinder containing three moles of a monatomic...Ch. 3 - A cylinder containing three moles of nitrogen gas...Ch. 3 - Two moles of a monatomic ideal gas such as oxygen...Ch. 3 - An insulated vessel contains 1.5 moles of argon at...Ch. 3 - One mole of an ideal monatomic gas occupies a...Ch. 3 - One mole of an ideal gas is initially in a chamber...Ch. 3 - A bullet of mass 10 g is traveling horizontally at...Ch. 3 - The insulated cylinder shown below is closed at...Ch. 3 - In a diesel engine, the fuel is ignited without a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Using the definitions in Eqs. 1.1 and 1.4, and appropriate diagrams, show that the dot product and cross produc...
Introduction to Electrodynamics
How does the change in kinetic energy of the ball in motion 1 compare to the change in kinetic energy of the ba...
Tutorials in Introductory Physics
kg/m3) reaches the 10-cm mark. You place an oak cube (density 900kg/m3) into the container. Each side of the cu...
College Physics
The reading on the spring scale just before the mass touches the lower spring.
College Physics: A Strategic Approach (3rd Edition)
Explain all answer clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desig...
The Cosmic Perspective Fundamentals (2nd Edition)
Determine the current in each resistor of the circuit shown in Fig. 26–77.
FIGURE 26–77 Problem 88.
Physics for Scientists and Engineers with Modern Physics
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Consider the process shown below. During steps AB and BC, 3600 J and 2400 J of heat, respectively, are added to the system. (a) Find the work done in each of the processes AB, BC, AD, and DC. (b) Find the internal energy change in processes AB and BC. (c) Find the internal energy difference between states C and A (d) Find the total heat added in the ADC process. (e) From the information given, can you find the heat added in process AD? Why or why not?arrow_forwardOf the following, which is not a statement of the second law of thermodynamics? (a) No heat engine operating in a cycle can absorb energy from a reservoir and use it entirely to do work, (b) No real engine operating between two energy reservoirs can be more efficient than a Carnot engine operating between the same two reservoirs, (c) When a system undergoes a change in state, the change in the internal energy of the system is the sum of the energy transferred to the system by heat and the work done on the system, (d) The entropy of the Universe increases in all natural processes, (e) Energy will not spontaneously transfer by heat from a cold object to a hot object.arrow_forwardWhich of the following is true for the entropy change of a system that undergoes a reversible, adiabatic process? (a) S 0 (b) S = 0 (c) S 0arrow_forward
- Consider cyclic processes completely characterized by each of the following net energy inputs and outputs. In each case, the energy transfers listed are the only ones occurring. Classify each process as (a) possible, (b) impossible according to the first law of thermodynamics, (c) impossible according to the second law of thermodynamics, or (d) impossible according to both the first and second laws. (i) Input is 5 J of work, and output is 4 J of work. (ii) Input is 5 J of work, and output is 5 J of energy transferred by heat. (iii) Input is 5 J of energy transferred by electrical transmission, and output is 6 J of work. (iv) Input is 5 J of energy transferred by heat, and output is 5 J of energy transferred by heat. (v) Input is 5 J of energy transferred by heat, and output is 5 J of work. (vi) Input is 5 J of energy transferred by heat, and output is 3 J of work plus 2 J of energy transferred by heat.arrow_forwardA heat pump used for heating shown in Figure P18.25 is essentially an air conditioner installed backward. It extracts energy from colder air outside and deposits it in a warmer room. Suppose the ratio of the actual energy entering the room to the work done by the devices motor is 10.0% of the theoretical maximum ratio. Determine the energy entering the room per joule of work done by the motor given that the inside temperature is 20.0C and the outside temperature is 5.00C. Figure P18.25arrow_forwardUse a PV diagram such as the one in Figure 22.2 (page 653) to figure out how you could modify an engine to increase the work done.arrow_forward
- Consider these scenarios and state whether work is done by the system on the environment (SE) or by the environment on the system (ES): (a) opening a carbonated beverage; (b) filling a flat tire; (c) a sealed empty gas can expands on a hot day, bowing out the walls.arrow_forwardConsider a transformation from point A to B in a two-step process. First, the pressure is lowered from 3 MPa at point A to a pressure of 1 MPa, while keeping the volume at 2 L by cooling the system. The state reached is labeled C. Then the system is heated at a constant pressure to reach a volume of 6 L in the state B. (a) Find the amount of work on the ACB path. (b) Find the amount of heat exchanged by the system when it goes from A to B on the ACB path. (c) the change in the internal energy when the AB process occurs adiabatically with the AB change though the two-step process on the ACB path.arrow_forwardConsider the cyclic process depicted in Figure P17.28. If Q is negative for the process BC and Eint is negative for the process CA, what are the signs of Q, W, and Eint that are associated with each of the three processes?arrow_forward
- A multicylinder gasoline engine in an airplane, operating at 2.50 103 rev/min, takes in energy 7.89 103 J and exhausts 4.58 103 J for each revolution of the crankshaft. (a) How many liters of fuel does it consume in 1.00 h of operation if the heat of combustion of the fuel is equal to 4.03 107 J/L? (b) What is the mechanical power output of the engine? Ignore friction and express the answer in horsepower. (c) What is the torque exerted by the crankshaft on the load? (d) What power must the exhaust and cooling system transfer out of the engine?arrow_forward(a) How much heat transfer occurs from 20.0 kg of 90.0C water placed in contact with 20.0 kg of 10.0C water, producing a final temperature of 50.0C ? (b) How much work could a Carnot engine do with this heat transfer, assuming it operates between two reservoirs at constant temperatures of 90.0C and 10.0C ? (c) What increase in entropy is produced by mixing 20.0 kg of 90.0C water with 20.0 kg of 10.0C water? (d) Calculate the amount of work made unavailable by this mixing using a low temperature of 10.0C, and compare it with the work done by the Garnet engine. Explicitly show how you follow the steps in the Problem-Solving Strategies for Entropy. (e) Discuss how everyday processes make increasingly more energy unavailable to do work, as implied by this problem.arrow_forwardA refrigerator has 18.0 kJ of work done on it while 115 kJ of energy is transferred from inside its interior. What is its coefficient of performance? (a) 3.40 (b) 2.80 (c) 8.90 (d) 6.40 (e) 5.20arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY