
Concept explainers
(a)
Interpretation: The Haworth projection for
Concept introduction: The structural representation of sugar molecule in cyclic form is known as Haworth projection. Sugar molecule that has six-membered-ring is known as pyranose and sugar molecule that has five-membered-ring is called furanose.
Answer to Problem 28.45P
The Haworth projection for
Explanation of Solution
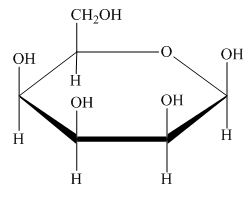
The structure of D-talose is,
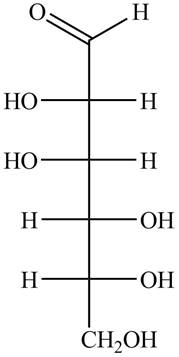
Figure 1
The steps for the conversion of Fischer projection of D-talose into Haworth projection is as follow:
Step-1 Talopyranose ring is formed by the attack of
Step 2 The Haworth projection of D-talopyranose,
Step 3 In the beta-form, the substituents
Step-4 The substituents which are present on the right side in the Fischer projection are drawn on the below the ring in the Haworth projection. Similarly, the substituents which are present on the left side in the Fischer projection are drawn on the above the ring in the Haworth projection.
The conversion of D-talose into
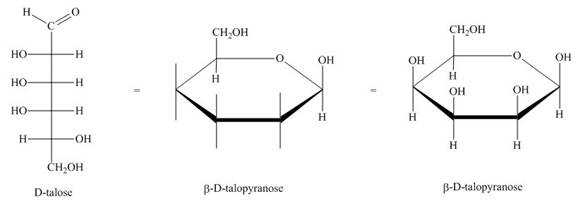
Figure 2
The Haworth projection for
(b)
Interpretation: The Haworth projection for
Concept introduction: The structural representation of sugar molecule in cyclic form is known as Haworth projection. Sugar molecule that has six-membered-ring is known as pyranose and sugar molecule that has five-membered-ring is called furanose.
Answer to Problem 28.45P
The Haworth projection for
Explanation of Solution
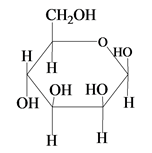
The structure of D-mannose is,
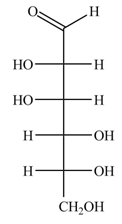
Figure 3
The steps for the conversion of Fischer projection of D-mannose into Haworth projection is as follow:
Step-1 Mannosepyranose ring is formed by the attack of
Step 2 The Haworth projection of D-mannosepyranose,
Step 3 In the beta-form, the substituents
Step-4 The substituents which are present on the right side in the Fischer projection are drawn on the below the ring in the Haworth projection. Similarly, the substituents which are present on the left side in the Fischer projection are drawn on the above the ring in the Haworth projection.
The conversion of D-mannose into D-mannosepyranose is shown below.
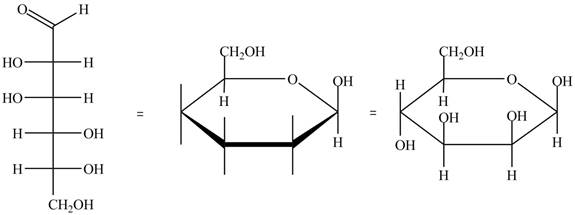
Figure 4
The Haworth projection for
(c)
Interpretation: The Haworth projection for
Concept introduction: The structural representation of sugar molecule in cyclic form is known as Haworth projection. Sugar molecule that has six-membered-ring is known as pyranose and sugar molecule that has five-membered-ring is called furanose.
Answer to Problem 28.45P
The Haworth projection for
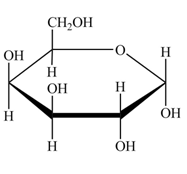
Explanation of Solution
The structure of D-galactose is,
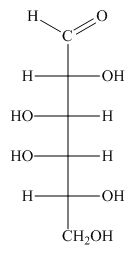
Figure 5
The steps for the conversion of Fischer projection of D-galactose into Haworth projection is as follow:
Step-1 Galactopyranose ring is formed by the attack of
Step 2 The Haworth projection of D-galactopyranose,
Step 3 In the alpha-form, the substituents
Step-4 The substituents which are present on the right side in the Fischer projection are drawn on the below the ring in the Haworth projection. Similarly, the substituents which are present on the left side in the Fischer projection are drawn on the above the ring in the Haworth projection.
The conversion of D-galactose into D-galactopyranose is shown below.
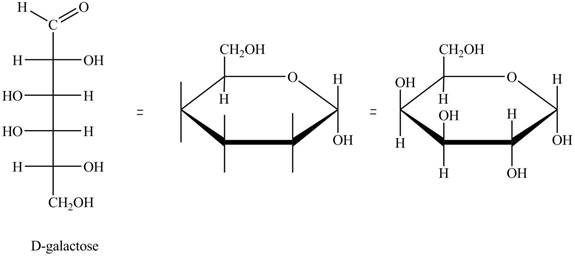
Figure 6
The Haworth projection for
(d)
Interpretation: The Haworth projection for
Concept introduction: The structural representation of sugar molecule in cyclic form is known as Haworth projection. Sugar molecule that has six-membered-ring is known as pyranose and sugar molecule that has five-membered-ring is called furanose.
Answer to Problem 28.45P
The Haworth projection for
Explanation of Solution
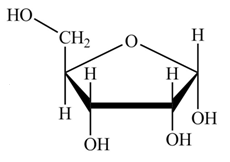
The structure of D-ribose is,
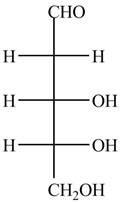
Figure 7
The steps for the conversion of Fischer projection of D-ribose into Haworth projection is as follow:
Step-1 Ribofuranose ring is formed by the attack of
Step 2 The Haworth projection of D-ribofuranose,
Step 3 In the alpha-form, the substituents
Step-4 The substituents which are present on the right side in the Fischer projection are drawn on the below the ring in the Haworth projection. Similarly, the substituents which are present on the left side in the Fischer projection are drawn on the above the ring in the Haworth projection.
The conversion of D-ribose into D-ribofuranose is shown below.
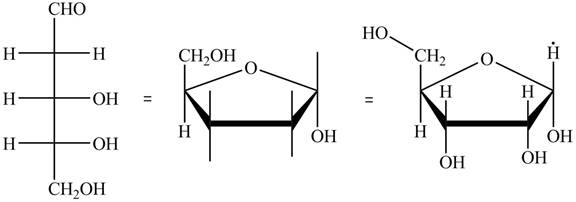
Figure 8
The Haworth projection for
(e)
Interpretation: The Haworth projection for
Concept introduction: The structural representation of sugar molecule in cyclic form is known as Haworth projection. Sugar molecule that has six-membered-ring is known as pyranose and sugar molecule that has five-membered-ring is called furanose.
Answer to Problem 28.45P
The Haworth projection for
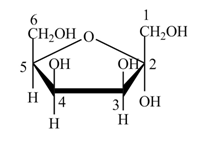
Explanation of Solution
The structure of D-tagatose is,
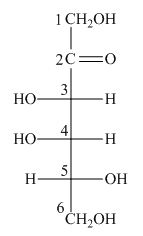
Figure 9
The steps for the conversion of Fischer projection of D-tagatose into Haworth projection is as follow:
Step-1 Tagatofuranose ring is formed by the attack of
Step 2 The Haworth projection of D-tagatofuranose,
Step 3 In the alpha-form, the substituents
Step-4 The substituents which are present on the right side in the Fischer projection are drawn on the below the ring in the Haworth projection. Similarly, the substituents which are present on the left side in the Fischer projection are drawn on the above the ring in the Haworth projection.
The conversion of D-tagatose into D-tagatofuranose is shown below.
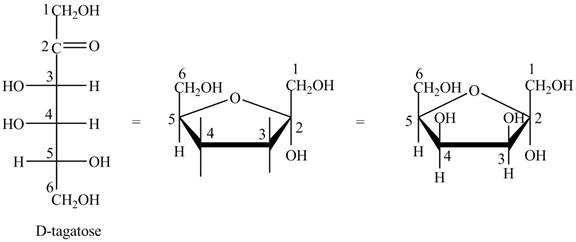
Figure 10
The Haworth projection for
Want to see more full solutions like this?
Chapter 28 Solutions
Organic Chemistry
- Answer the following questions using the Haworth structure drawn below. Но. CH2 HO, ОН OH ОН a. Identify this monosaccharide. b. Is this the a or ß isomer. c. In solution the cyclic structure above can open and close to form the other isomer. Which structure below is the other isomer that is present in solution? I. II. III. CH2OH O OH CH2OH но -СН, о OH OH OH но CH2 -OH OH ÓH ÓH OHarrow_forwardThe products of the hydrolysis of a fat include fatty acids and a. aldehydes b. glycerol c. esters d. more than one response is correct Which of the following has an amino alcohol (rather than glycerol) backbone in its structure? a. phosphoglyceride b. wax c. sphingolipid d. soap Which of the following compounds is significantly soluble in water? a. 2,5-dimethyloctane b. 2,5-dimethyl-3-octene c. 2-methyl-5-phenyloctane d. all are significantly soluble e. none are significantly soluble What reactant and catalyst is(are) needed to change ethene into ethane? a. H2, Pt b. H2O, H2SO4 c. HCl d. heat, pressurearrow_forward1. Write Haworth projection formulas for such substances: 4.1 B-D-glucofuranose; 4.2 a-D-fructopyranose; 4.3 saccharose. 1.4 In the formulas a and b show the hemi-acetal hydroxyl. 2. Write: why lactose is a reducing disaccharide. 3. Write the equations of reactions: 3.1 glucose +Cu(OH):(heat)→; 3.2 glucose +HNO,→; 3.3 и-D-glucopyranose +CH,1—; 3.4 a-D-glucopyranose +(CH,CO),O→; 3.5 lactose hydrolysis; 3.6 glucuronic acid formation from glucose. 4. Describe the signs of reactions: 4.1 iodine starch reaction; 4.2 Selivanov's reactions for the fructose; 4.3 glucose reactions with Cu(OH), with heating.arrow_forward
- 1. Write Haworth projection formulas for such substances: 4.1 B-D-glucofuranose; 4.2 a-D-fructopyranose; 4.3 saccharose. 1.4 In the formulas a and b show the hemi-acetal hydroxyl. 2. Write: why lactose is a reducing disaccharide. 3. Write the equations of reactions: 3.1 glucose +Cu(ОН),(heat) —; 3.2 glucose +HNO,→; 3.3 a-D-glucopyranose +CH,I→; 3.4 a-D-glucopyranose +(CH;CO);O→; 3.5 lactose hydrolysis; 3.6 glucuronic acid formation from glucose. 4. Describe the signs of reactions: 4.1 iodine starch reaction; 4.2 Selivanov's reactions for the fructose; 4.3 glucose reactions with Cu(OH); with heating.arrow_forwardIdentify the major class of each lipid from its components. a. fatty acids + glycerol b. fatty acids + sphingosine + a carbohydrate c. fatty acids + high-molecular-weight alcohols other than glycerol d. glycerol + fatty acids + phosphate group + choline e. fatty acids + sphingosine + phosphate group + a nitrogen compoundarrow_forward10 Which of the following are chiral C-atoms in the chemical structure of this opioid analgesic? Attachments R¹0 R²0 A. a B. b ⒸC.C O D.d (b) (a) N— (c) E. a and b F. b and c G. a and c ⒸH. a, b and c are all chiral C-atomsarrow_forward
- Which of the following is FALSE? a. D-altrose and D-talose have the same osazones. b. D-glucose and D-galactose have different osazones. c. Trioses and tetroses cannot form the furfural derivatives because of their inability to form the 5-membered ring. d. Barfoed’s reagent, a weaker oxidizing agent than Benedict’s reagent, can only oxidize monosaccharides.arrow_forward81. Indicate beloging to glycofrangulin. I. aglycone part is frangulin II. it is aglycone III. spasmolytic effect IV. formed as a result of oxidation of frangularoside V. taken from Aloe VI. there are 4 rhamnose molecules VII. it is biglycoside VIII. taken from Alder buckthorn IX. alizarin derivate X. glycoside A) III; VII; IX B) III; IV; VII; IX C) II; IV; VIII; IX; X D) I; IV; VII; VIII; X E) I; III; V; VII; VIIarrow_forwardChemistry 1. Fill in the blanks with the appropriate word or words: B-Lactam acts by inhibition They are also liberating and thus exert their rapid bactericidal effect. 2. Which sentence is correct and which is incorrect? If it is incorrect, write the correct sentence a. ß - Lactams cause transpeptidation in glycopeptides. b. ß-lactams act in the third stage of murein complex synthesis. c. ß-lactams inhibit protein synthesis in bacteria. d. Bacitracin prevents the dephosphorylation of the carrier molecule of the peptidoglycan subunit. e. Cycloserine is involved in the second stage of peptidoglycan synthesis.arrow_forward
- 14. Which method is NOT typically used to break down lipids into their components? A. alkaline hydrolysis B. acid hydrolysis C. phospholipases D. strong oxidation 15. Mass spectrometry: A. cannot be used with lipids other than fatty acids. B. can determine the mass but not the identify of a lipid. C. can be used to identify individual lipids in complex mixtures. D. cannot determine the locations of double bonds in a fatty acid.arrow_forwardGuar gum is a complex polysaccharide used by the food industry as a stabilizer in salad dressings, ice cream and cream cheese. HO HO- OH linkage A sugar chain HO- 11 OH OH 0. HO n HO sugar off the chain OH o O linkage B HO- OH HO- O a. Linkage A is 1,5 a glycosidic linkage O b. Linkage A is 1,4 a glycosidic linkage O c. Linkage A is 1,5 ß glycosidic linkage O d. Linkage A is 1,4 ß glycosidic linkage of marrow_forwardWhat is the function of lyases? a. to catalyze redox reactions b. They catalyze reactions in which H₂O, NH3 and CO2 groups are removed c. They catalyze hydrolysis reactions. D. transfer active groupsarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,


