
Concept explainers
Draw short segments of the
(a)
Interpretation:
The short segment of polymer obtained from the given monomer has to be drawn and also have to indicate whether it is a chain growth or step growth polymer.
Answer to Problem 25P
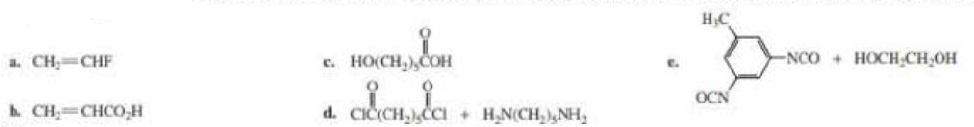
The short segment of given monomer is shown below and the monomer undergoes chain growth polymerization.
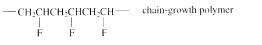
Explanation of Solution
Given the monomer is
The short segment of polymer obtained from this monomer is given below.
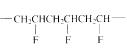
The polymer formed is a Chain growth polymer because monomer addition occurs at the end of the chain.
(b)
Interpretation:
The short segment of polymer obtained from the given monomer has to be drawn and also have to indicate whether it is a chain growth or step growth polymer.
Answer to Problem 25P
The short segment of given monomer is shown below and the monomer undergoes chain growth polymerization.
Explanation of Solution
Given the monomer is
The short segment of polymer obtained from this monomer is given below.
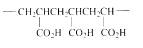
The polymer formed is a Chain growth polymer because monomer addition occurs at the end of the chain.
(c)
Interpretation:
The short segment of polymer obtained from the given monomer has to be drawn and also have to indicate whether it is a chain growth or step growth polymer.
Answer to Problem 25P
The short segment of given monomer is shown below and the monomer undergoes step growth polymerization.
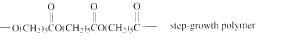
Explanation of Solution
Given the monomer is
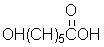
The short segment of polymer obtained from this monomer is given below.
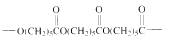
The polymer formed is a Step growth polymer because monomer addition occurs not at the end of the chain and these polymers are formed by combining monomers by removing small molecules of water or alcohol.
(d)
Interpretation:
The short segment of polymer obtained from the given monomer has to be drawn and also have to indicate whether it is a chain growth or step growth polymer.
Answer to Problem 25P
The short segment of given monomer is shown below and the monomer undergoes step growth polymerization.
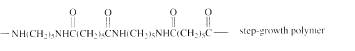
Explanation of Solution
Given the monomer is
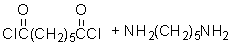
The short segment of polymer obtained from this monomer is given below.

The polymer formed is a step growth polymer because monomer addition occurs not at the end of the chain and these polymers are formed by combining monomers by removing small molecules of water or alcohol.
(e)
Interpretation:
The short segment of polymer obtained from the given monomer has to be drawn and also have to indicate whether it is a chain growth or step growth polymer.
Answer to Problem 25P
The short segment of given monomer is shown below and the monomer undergoes step growth polymerization.
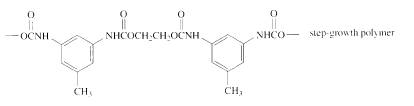
Explanation of Solution
Polymers are formed from linking small units called monomers and they are classified into Chain growth polymers and Step growth polymers.
Given the monomer is
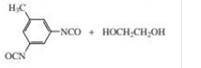
The short segment of polymer obtained from this monomer is given below.
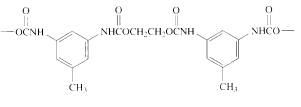
The polymer formed is a Step growth polymer because monomer addition occurs not at the end of the chain and these polymers are formed by combining monomers by removing small molecules of water or alcohol.
Want to see more full solutions like this?
Chapter 27 Solutions
EBK ORGANIC CHEMISTRY
- Viscosity of a liquid related to the activation energy.arrow_forwardVibrational contributions to internal energy and heat capacity1) are temperature independent2) are temperature dependentarrow_forwardThe approximation of calculating the partition function by integration instead of the summation of all the energy terms can only be done if the separation of the energy levels is much smaller than the product kT. Explain why.arrow_forward
- Explain the meaning of: the electron partition function is equal to the degeneracy of the ground state.arrow_forward28. For each of the following species, add charges wherever required to give a complete, correct Lewis structure. All bonds and nonbonded valence electrons are shown. a. b. H H H H H :0-C-H H H H-C-H C. H H d. H-N-0: e. H H-O H-O H B=0 f. H—Ö—Ñ—Ö—H Norton Private Barrow_forwardAt 0oC and 1 atm, the viscosity of hydrogen (gas) is 8.55x10-5 P. Calculate the viscosity of a gas, if possible, consisting of deuterium. Assume that the molecular sizes are equal.arrow_forward
- Indicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forwardIndicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forwardDraw the skeletal structure of the alkane 4-ethyl-2, 2, 5, 5- tetramethylnonane. How many primary, secondary, tertiary, and quantenary carbons does it have?arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





