
Concept explainers
(a)
Interpretation:
The complex ion
(a)
Explanation of Solution
Complex ion
Given complex ion is
Given complex contains
Electronic configuration of cobalt is given as shown below;
Electronic configuration of
Therefore, there are six electrons in
Complex ion
Given complex ion is
Given complex contains
Electronic configuration of cobalt is given as shown below;
Electronic configuration of
Therefore, there are seven electrons in
Comparing the
Therefore, the given statement is false.
(b)
Interpretation:
The complex ion
(b)
Explanation of Solution
Complex ion
Given complex ion is
Given complex contains
Electronic configuration of cobalt is given as shown below;
Electronic configuration of
Therefore, there are six electrons in
Complex ion
Given complex ion is
Given complex contains
Electronic configuration of cobalt is given as shown below;
Electronic configuration of
Therefore, there are seven electrons in
Comparing the
Therefore, the given statement is false.
(c)
Interpretation:
The complex ion
(c)
Explanation of Solution
Complex
Given complex ion is
Given complex contains
In the octahedral complex, the
Electronic configuration of cobalt is given as shown below;
Electronic configuration of
Therefore, there are six electrons in
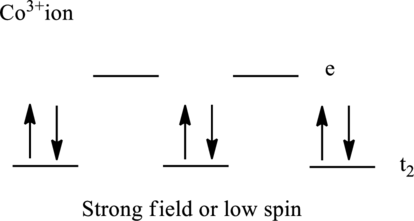
Therefore, there are no unpaired electrons in
Complex
Given complex ion is
Given complex contains
In the octahedral complex, the
Electronic configuration of cobalt is given as shown below;
Electronic configuration of
Therefore, there are seven electrons in
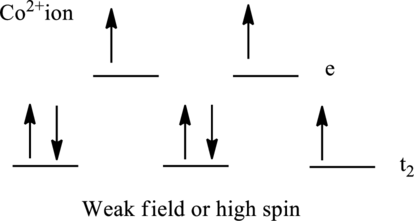
Therefore, there are three unpaired electrons in
Thus the given statement is false.
(d)
Interpretation:
The complex ion
(d)
Explanation of Solution
Complex
Given complex ion is
Given complex contains
In the octahedral complex, the
Electronic configuration of cobalt is given as shown below;
Electronic configuration of
Therefore, there are six electrons in
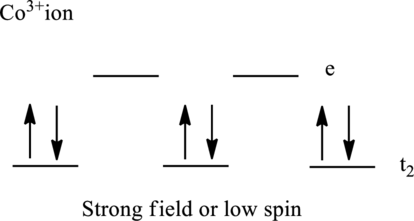
Therefore, there are no unpaired electrons in
Complex
Given complex ion is
Given complex contains
In the octahedral complex, the
Electronic configuration of cobalt is given as shown below;
Electronic configuration of
Therefore, there are seven electrons in
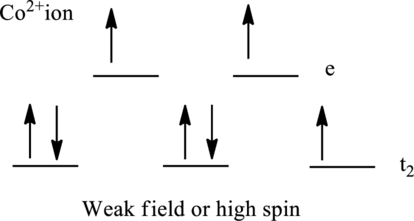
Therefore, there are three unpaired electrons in
Thus the given statement is true.
Want to see more full solutions like this?
Chapter 26 Solutions
General Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





