
Concept explainers
Devise a synthesis of each of the following compounds. Besides inorganic reagents, you may use hydrocarbons and halides having
a. c.
c. e.
e.
b.![]() d.
d.![]() f.
f.
(a)
Interpretation: The synthesis of the given compound is to be devised.
Concept introduction: Lithium aluminum hydride is a strong reducing agent. Treatment of carbonyl compounds with lithium aluminum hydride yields alcohols. It reduces ketone into secondary alcohol.
Answer to Problem 26.49P
The synthesis of the given compound is,
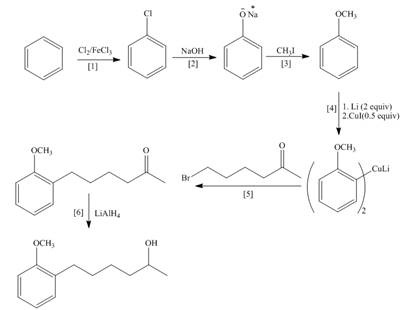
Explanation of Solution
The synthesis of given compound is follows:
Step-1 Chlorination of benzene takes place in the presence of ferric chloride and gives chlorobenzene.
Step-2 Chlorobenzene is treated with base and undergoes nucleophilic substitution reaction and gives phenol.
Step-3 Phenol is treated with methyl iodide and gives anisole.
Step-4 Anisole is treated with
Step-5 Organocuprate compound is treated with
Step-6 The substituted product is treated with
The synthesis of the given compound is shown below.
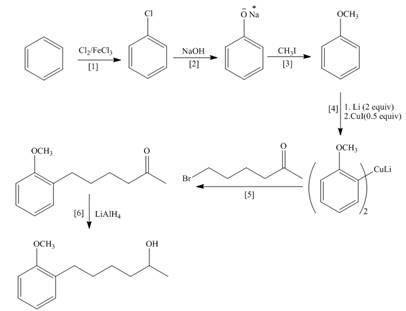
Figure 1
The synthesis of given compound is shown in Figure 1.
(b)
Interpretation: The synthesis of the given compound is to be devised.
Concept introduction: Gilman reagents are organometallic compounds which are a source of nucleophiles. These nucleophiles give
Answer to Problem 26.49P
The synthesis of the given compound is,
Explanation of Solution
The synthesis of the given compound is follows:
Step-1 Alkene is treated with
Step-2
Step-3The
Step-4 The compound obtained from step-4 is treated with
Step-5 The Wittig reagent is treated with acetone to give a desired product.
The synthesis of the given compound is shown below.

Figure 2
The synthesis of the given compound is shown in Figure 2.
(c)
Interpretation: The synthesis of the given compound is to be devised.
Concept introduction: Suzuki reaction is a type of substitution reaction. It involves the palladium coupling between organic halide with organoborane in the presence of base. It is a stereospecific reaction.
Answer to Problem 26.49P
The synthesis of the given compound is,
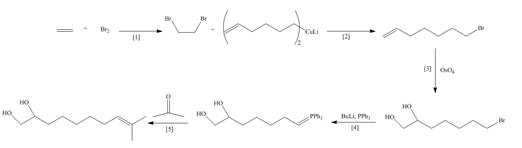
Explanation of Solution
The synthesis of the given compound is follows:
Step-1 Hydroboration of hex
Step-2 The
The synthesis of the given compound is shown below.
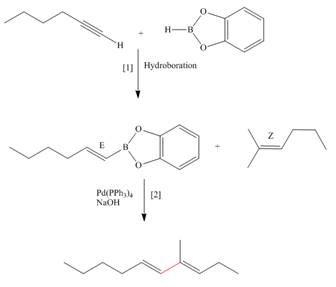
Figure 3
The synthesis of the given compound is shown in Figure 3.
(d)
Interpretation: The synthesis of the given compound is to be devised.
Concept introduction: Heck reaction is a type of substitution reaction. It involves the palladium coupling between aryl or vinyl halide with alkene which leads to the formation of trans alkene at less substituted carbon.
Answer to Problem 26.49P
The synthesis of the given compound is,
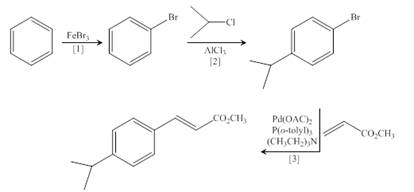
Explanation of Solution
The synthesis of the given compound is follows:
Step-1 Bromination of benzene takes place in the presence of ferric chloride and gives bromobenzene.
Step-2 Friedelcraft alkylation of bromobenzene takes place in the presence of isopropyl chloride.
Step-3The
The synthesis of the given compound is shown below.
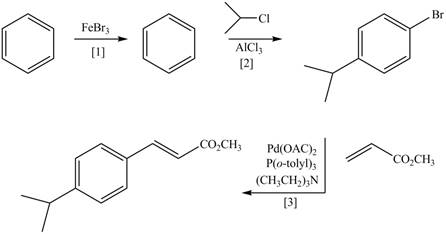
Figure 4
The synthesis of the given compound is shown in Figure 4.
(e)
Interpretation: The synthesis of the given compound is to be devised.
Concept introduction: Heck reaction is a type of substitution reaction. It involves the palladium coupling between aryl or vinyl halide with alkene which leads to the formation of trans alkene at less substituted carbon. The nonhalogenated cyclopropanes are synthesized by the treatment of an alkene with
Answer to Problem 26.49P
The synthesis of given compound is,
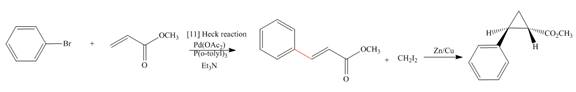
Explanation of Solution
The synthesis of given compound is as follows:
Step-1 Bromobenzene is treated with
Step-2 The compound formed in step-1 undergoes Simmons-Smith reaction and forms a desired product.
The synthesis of the given compound is shown below.
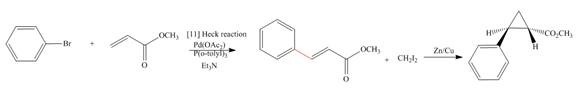
Figure 5
The synthesis of given compound is shown in Figure 5.
(f)
Interpretation: The synthesis of given compound is to be devised.
Concept introduction: Heck reaction is a type of substitution reaction. It involves the palladium coupling between aryl or vinyl halide with alkene which leads to the formation of trans alkene at less substituted carbon.
Answer to Problem 26.49P
The synthesis of given compounds is,
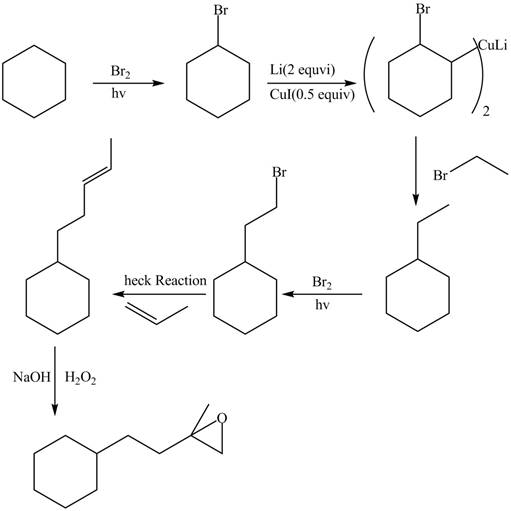
Explanation of Solution
The synthesis of given compounds is as follows:
Step-1 Cyclohexane is treated with
Step-2 The brominated compound then treated with
Step-3 The organocuprate compound then treated with organic bromide to form ethylcyclohexane.
Step-4 Then ethylcyclohexane undergoes heck reaction in the presence of 1-propene to form substituted alkene compound.
Step-5 The substituted alkene compound reacts with hydrogen peroxide in the sodium hydroxide to form the desired oxirane product.
The synthesis of the given compound is shown below.
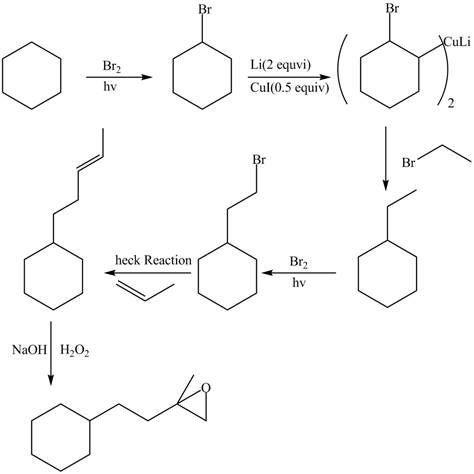
Figure 6
The synthesis of given compound is shown in Figure 6.
Want to see more full solutions like this?
Chapter 26 Solutions
Organic Chemistry
- (12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- Don't used Ai solutionarrow_forwardIndicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are falsearrow_forward(f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
- 1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
