
Interpretation:
Tetrapeptide obtained when two molecules of Cys-Phe are joined by a disulfide bridge need to be drawn.
Concept introduction:
Disulfide linkages are formed when two thiol molecules undergo oxidation. In case of amino acids only cysteine has a thiol group in it. Therefore cysteine can involve in disulfide bridge formation. If the disulfide bridge is formed within the peptide a cyclic structure is obtained due to intermolecular oxidation of thiol. If the disulfide bridge is formed between two molecules of thiol, then the disulfide is formed due to intramolecular oxidation of thiol. The general scheme of forming disulfide linkages can be shown as,
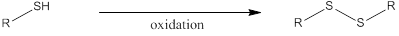
To draw: the tetrapeptide formed when Cys-Phe is joined by disulfide bridge.
Want to see the full answer?
Check out a sample textbook solution
Chapter 25 Solutions
EBK ORGANIC CHEMISTRY STUDENT SOLUTION
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





