
Concept explainers
(a)
Interpretation:
The role of the process of diffusion in the transport of gases through the body needs to be explained.
Concept Introduction:
The gasses are transported from the lungs to the whole body and this transfer of gases in the lungs takes place by the process of diffusion. The gases in the lungs diffuse from their region of high pressure to their region of low pressure and are either taken throughout the body or exhale out depending upon the gas.
(b)
Interpretation:
The role of carbaminohemoglobin in the transport of gases throughout the body needs to be explained.
Concept Introduction:
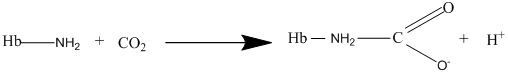
Other than diffusion, there are other methods for the transport of gases. The carbaminohemoglobin is one of the methods of transport for the carbon dioxide gas where the carbon dioxide binds with hemoglobin to form carbaminohemoglobin as per the following equation:
(c)
Interpretation:
The role of alveoli in the transfer of gases throughout the body needs to be explained.
Concept Introduction:
Alveoli are the air sacs in the lungs which basically increases the surface area capacity of the lungs and these alveoli are the sites of the gases transfer from the atmosphere to the blood in the body.
(d)
Interpretation:
The role of carbonic anhydrase in the transfer of gases throughout the body needs to be explained.
Concept Introduction:
The transfer of carbon dioxide also takes place by means of carbonic anhydrase. The carbonic anhydrase converts to carbonic acid

Want to see the full answer?
Check out a sample textbook solution
Chapter 25 Solutions
General, Organic, & Biological Chemistry
- Suppose the pressure in the esophagus is -1.8 mm Hg while that in the stomach is +21 mm Hg. To what height, in centimeters, could stomach fluid rise in the esophagus, assuming it has a density of 1.10g/mL ?arrow_forwardExplain the role of surfactants in the lungs and Explain why premature babies often have respiratory issuesarrow_forwardThe process of lysing occurs when the concentration outside a cell is and is said to be lower in concentration; hypotonic higher in concentration; hypertonic higher in concentration; hypotonic lower in concentration; hypertonicarrow_forward
- If a deep-sea diver ascends to the surface (1 atm) from a pressure of 5 atm, the volume of the dissolved gas bubbles in the diver's blood will increase by a factor of during the ascent. 10 O 5 15 1arrow_forwardHow many parts per million (ppm) is 1mg/L? 1ppm O 10ppm O 100ppm O 1000ppmarrow_forward19arrow_forward
- When the partial pressure of O2 in venous blood is 30 torr, the saturation of myoglobin with O2 is ______ while the saturation of hemoglobin with O2 is ______. a. 0.55, 0.91 b. 0.91, 0.55 c. 2.8 torr, 26 torr. d. 0.91, 0.97 e. none of the above please show your work, thanks! Please don't provide handwriting solutionarrow_forwardAccording to the values of a bag of nouns one serving 48 grams contains 9 grams of fat 2 grams of protein and 34 grams of carbohydrates determine the total number of dietary calories according to the Atwater factors.arrow_forwardexplain the answerarrow_forward
- At 0 C and 1.00 atm, as much as 0.70 g of O2 can dissolve in 1 L of water. At 0 C and 4.00 atm, how many grams of O2 dissolve in 1 L of water?arrow_forwardAccumulation of cholesterol leads to the hardening of the arteries. This is called: a.vasoconstriction. b.venipuncture. c.atherosclerosis. d.hypertension.arrow_forward5-89 (Chemical Connections 5C) In a sphygmomanometer one listens to the first tapping sound as the constrictive pressure of the arm cuff is slowly released. What is the significance of this tapping sound?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





