
Concept explainers
(a)
Interpretation:
The product formed by the reaction of D-ribose with
Concept Introduction:
The reaction of
(a)
Explanation of Solution
The D-ribose is an aldopentose molecule. The carbonyl group present in D-ribose is
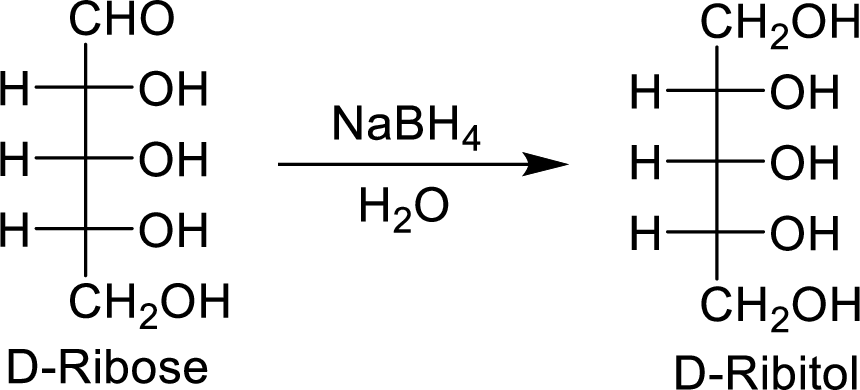
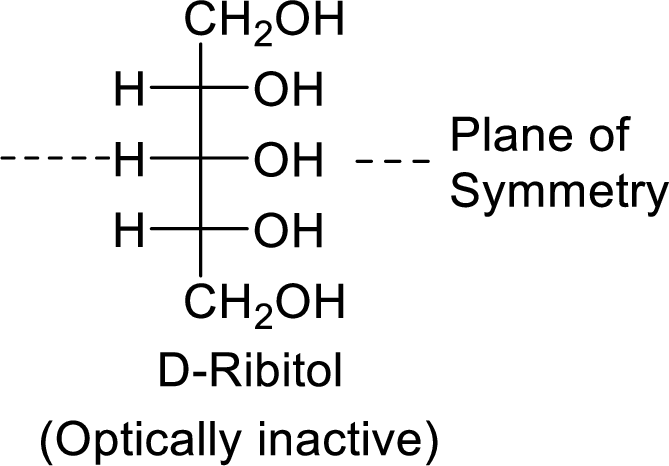
The D-ribitol is a meso compound and shows plane of symmetry. So, it is optically inactive.
(b)
Interpretation:
The product formed by the reaction of D-ribose with
Concept Introduction:
The reagent
(b)
Explanation of Solution
When the D-ribose molecule is made to react with
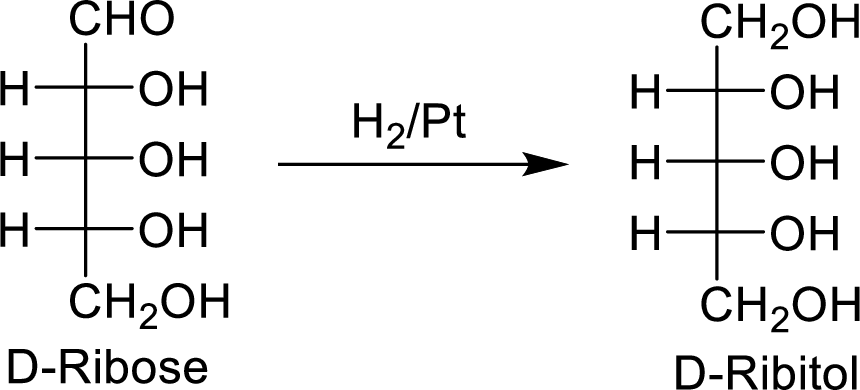
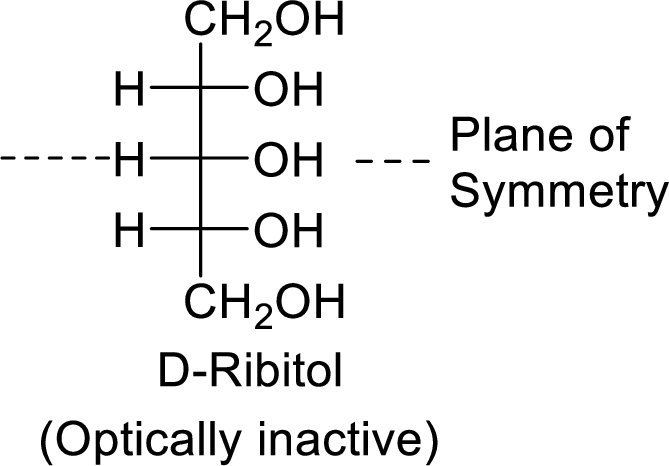
The D-ribitol is a meso compound and shows plane of symmetry. So, it is optically inactive.
(c)
Interpretation:
The product formed by the reaction of D-ribose with warm
Concept Introduction:
The reaction of warm
(c)
Explanation of Solution
The nitric acid (
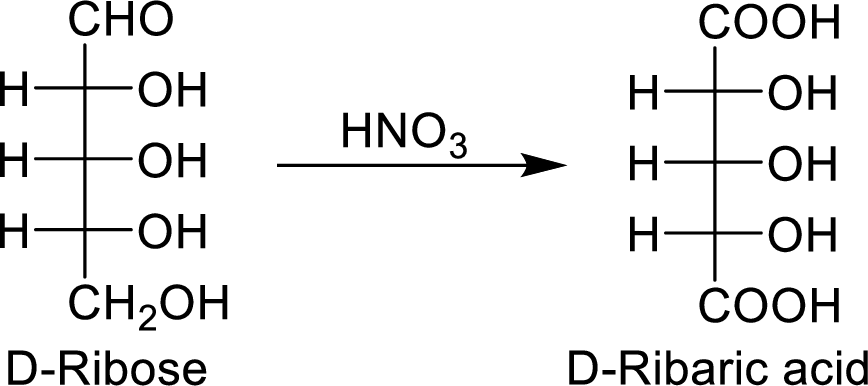
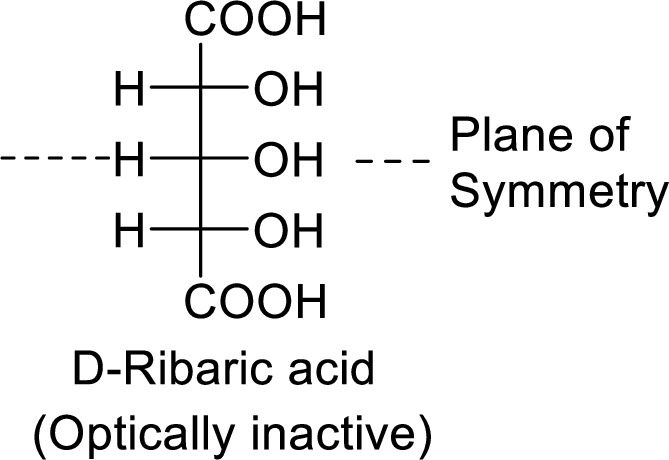
The product formed is a meso compound and shows plane of symmetry. So, it is optically inactive.
(d)
Interpretation:
The product formed by the reaction of D-ribose with
Concept Introduction:
The reaction of
(d)
Explanation of Solution
When the D-ribose reacts with
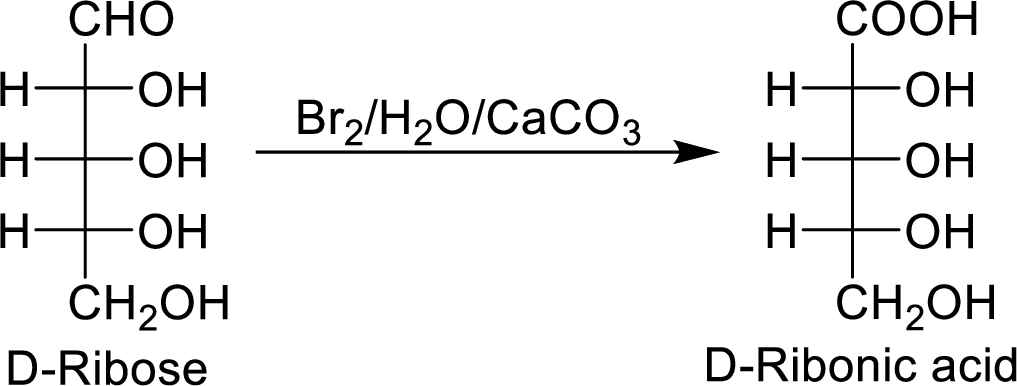
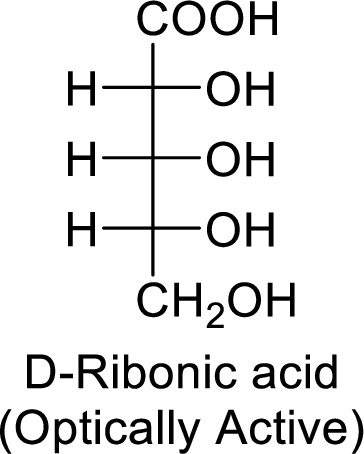
The D-ribonic acid is a chiral molecule and is optically active.
(e)
Interpretation:
The product formed by the reaction of D-ribose with
Concept Introduction:
The reaction of
(e)
Explanation of Solution
The D-ribose molecule consumes
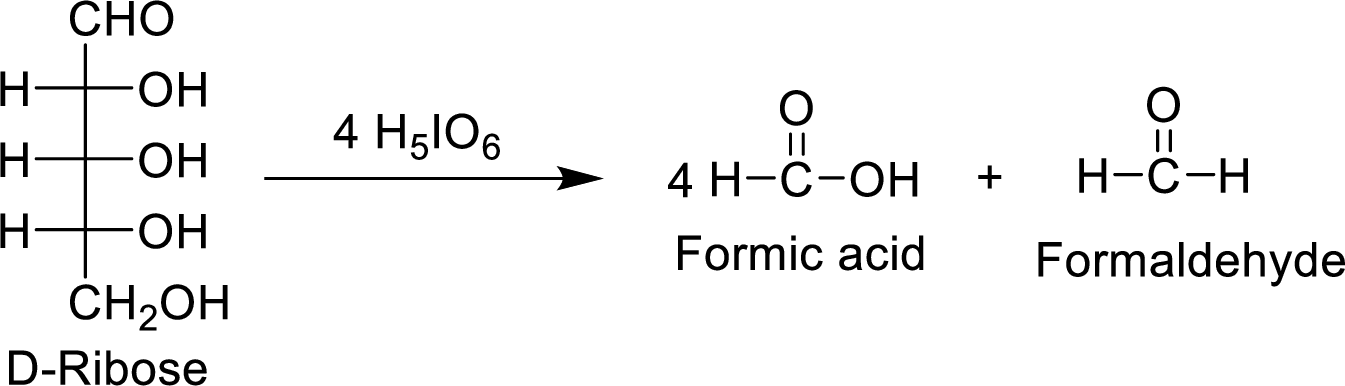
The products formed are achiral and are optically inactive.
(f)
Interpretation:
The product formed by the reaction of D-ribose with aniline (
(f)
Explanation of Solution
The D-galactose molecule reacts with aniline molecule and the aldehyde group of carbohydrate is reacted with the
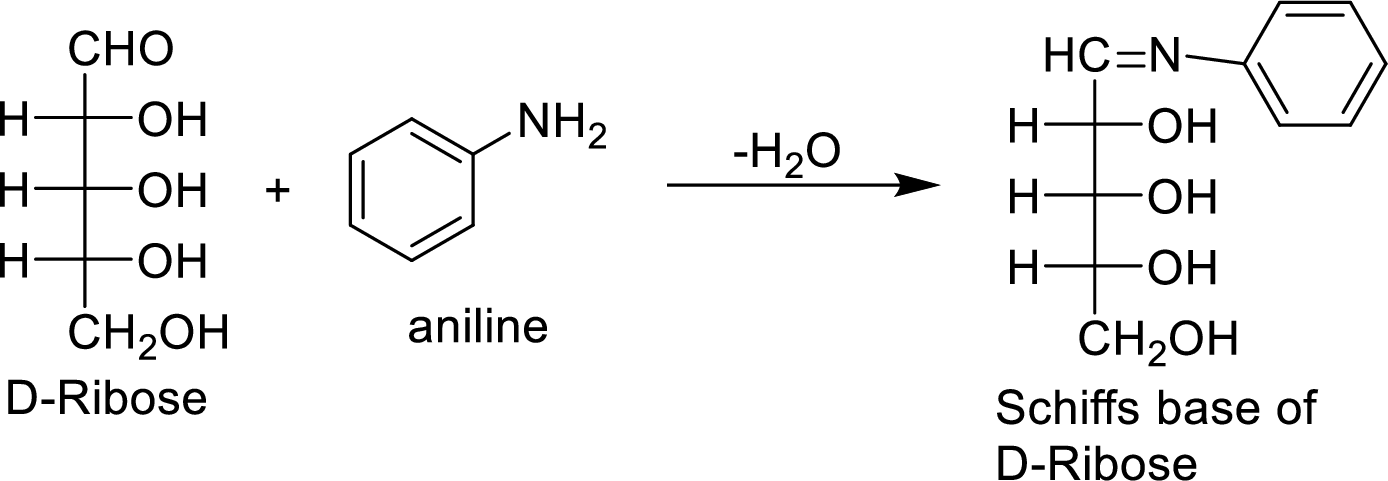
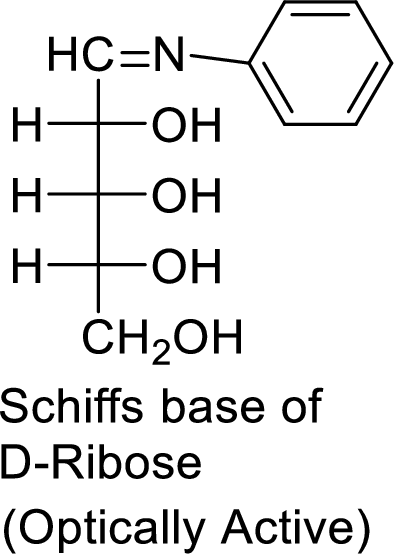
The product formed is chiral and optically active.
Want to see more full solutions like this?
Chapter 25 Solutions
OWLv2 with MindTap Reader, 1 term (6 months) Printed Access Card for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Monosaccharide A is a diastereomer of d-lyxose. Treatment of A with nitric acid forms an optically inactive aldaric acid. A undergoes a Kiliani-Fischer synthesis to form B and C. B is oxidized by nitric acid to an optically active aldaric acid, and C is oxidized to an optically inactive aldaric acid. Wohl degradation of A forms D, which is oxidized by nitric acid to an optically inactive aldaric acid. Wohl degradation of D forms a d-aldotriose. Identify A, B, C, and D.arrow_forward(a) Figure 23-2 shows that the degradation of d-glucose gives d-arabinose, an aldopentose.Arabinose is most stable in its furanose form. Draw d-arabinofuranose.(b) Ribose, the C2 epimer of arabinose, is most stable in its furanose form. Drawd-ribofuranose.arrow_forwardDraw the structure of: (a) a polysaccharide formed by joining D-mannose units in 1->4-ß-glycosidic linkages; (b) a polysaccharide formed by joining D-glucose units in 1->6-a-glycosidic linkages. The polysaccharide in (b) is dextran, a component of dental plaque.arrow_forward
- Predict the products obtained when d-galactose reacts with each reagent. (f) excess Ac2O and pyridine (g) excess CH3 I, Ag2Oarrow_forwardWhich D-aldopentoses are reduced to optically inactive alditols using NaBH4, CH3OH?arrow_forwardGiven the following structure of aldose, (a) how many chiral carbons are there? (b) is it a reducing sugar? and (c) is it an L or D sugar?arrow_forward
- Compound A is a D-aldopentose that can be oxidized to an optically inactive aldaric acid B. On Kiliani-Fischer chain extension, A is converted into C and D; C can be oxidized to an optically active aldaric acid E, but D is oxidized to an optically inactive aldaric acid F. What is the structure of compound F? • Use the wedge/hash bond tools to indicate stereochemistry where it exists. You do not have to explicitly draw H atoms. If a group is achiral, do not use wedged or hashed bonds on it. • Show stereochemistry in a meso compound. • Do not include lone pairs in your answer. They will not be considered in the grading.arrow_forwardThere are three (3) vials labeled A, B, and C known to contain the following monosaccharides. All three samples reduce Tollens and Fehling. By oxidation with dilute HNO3 an optically active aldaric acid is obtained for sample A and the remaining two give products without optical activity. When the three samples were subjected to an alkaline medium, it was observed that, after a certain time, samples A and C reached the same value of the specific rotation [α]. Select the RIGHT alternative: (a) Sample A is Galactose. (b) Sample B is Alosa. (c) Samples A and C are not related to each other by an epimerization process. (d) Sample C is Talose. (e) Samples B and C are epimers.arrow_forwardThere are four d-aldopentoses (Table 25.1). If each is reduced with NaBH4, which yield optically active alditols? Which yield optically inactive alditols?arrow_forward
- An unknown reducing disaccharide is found to be unaffected by invertase enzymes. Treatment with an a@galactosidasecleaves the disaccharide to give one molecule of d-fructose and one molecule of d-galactose. When the disaccharideis treated with excess iodomethane and silver oxide and then hydrolyzed in dilute acid, the products are2,3,4,6-tetra-O-methylgalactose and 1,3,4-tri-O-methylfructose. Propose a structure for this disaccharide, and give itscomplete systematic name.arrow_forwardThe following observations are obtained after a D-hexose was made to react with several reagents: (1) The reactions of a D-hexose with (a) to (d) below yields an aldaric acid (a) NH₂OH, (b) (CH3CO)₂O, NaOCOCH 3, and, (c) NaOCH3, and then, (d) HNO3, H₂O (2) HNO3 oxidation of the same D-hexose gives an aldaric acid. Predict the structures of the three (3) possible hexoses that can undergo the above reactions?arrow_forward(d) Use the diagram below to complete the cyclic alpha form of structure V (e) Circle the hemiacetal in cyclic alpha form of structure V. (f) Redraw the cyclic alpha form of structure V but replace the OH group on the anomericcarbon with a methoxy group. Is this modified monosaccharide a reducing sugar or anonreducing sugar?arrow_forward

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

