
(a)
Interpretation: The primary, secondary or tertiary amine in the given compound is to be classified.
Concept introduction:
In secondary amines, two of the hydrogen atoms of ammonia are replaced by alkyl or aryl group.
In tertiary amines, all hydrogen atoms of ammonia are replaced by alkyl or aryl group.
Answer to Problem 25.1P
Each amine in the given compound is classified as,
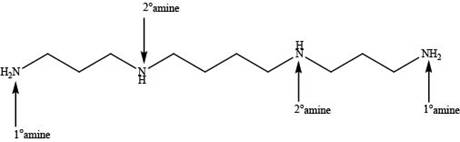
Explanation of Solution
The given compound is shown below.
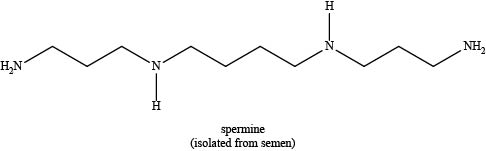
Figure 1
In spermine, four nitrogen atoms are present. Among these four nitrogen atoms, two are primary amines that contain one alkyl group on nitrogen atom and two are secondary amines that contain two alkyl groups on nitrogen atom as shown below.
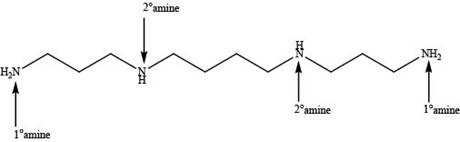
Figure 2
In spermine, primary and secondary amines are present as shown in Figure 2.
(b)
Interpretation: The primary, secondary or tertiary amine in the given compound is to be classified.
Concept introduction: Amines are nitrogen-containing organic compounds. The general formula of amines is
In secondary amines, two of the hydrogen atoms of ammonia are replaced by alkyl or aryl group.
In tertiary amines, all hydrogen atoms of ammonia are replaced by alkyl or aryl group.
Answer to Problem 25.1P
Each amine in the given compound is classified as,
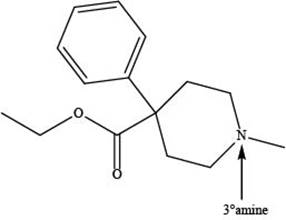
Explanation of Solution
The given compound is shown below.
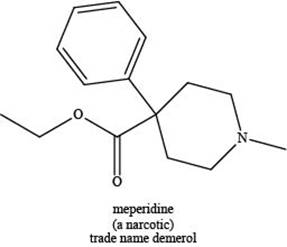
Figure 3
Meperidine contains one nitrogen atom bonded to three alkyl groups. Hence, it is a tertiary amine as shown below.

Figure 4
In meperidine, tertiary amine is present as shown in Figure 4.
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry-Package(Custom)
- Classify each amine in the following compounds as 1°, 2°, or 3°. NH2 a. H,N b. CH,CH20. N-CH, spermine (isolated from semen) meperidine (a narcotic) Trade name: Demerolarrow_forwardThe hydrolysis of an amide in acidic conditions forms A. a carboxylate salt and an alcohol B. a carboxylate salt and an amine C. an alcohol and an amine salt (an ammonium ion) D. a carboxylic acid and an amine salt (an ammonium ion)arrow_forwardWhich of the following is an amine? Select one: a. HCONH2 b. CH3COCH3 c. CH3F d. CH3NH2arrow_forward
- What amine is formed by reduction of each amide?arrow_forwardselect two. Which statements correspond to: ESCITALOPRAM (antidepressant) a. It induces tranquility and somnolence to the patient. b. An agent that regulates re-uptake of serotonin in the brain. c. Its structure has benzonitrile, flurobenzene and a tertiary amine. d. An agent that is indicated to patients with hives and dermatitis.arrow_forward1. What amine and what carbonyl compound are required to make the following molecule: N.arrow_forward
- What are the functional groups present in this antibacterial antibiotic? A. Nitro, phenyl, amine, carbonyl, hydroxyl B. Nitro, phenyl, amine, carbonyl, C. Nitro, phenyl, amine, hydroxyl D. Nitro, phenol, amine, carbonyl, hydroxyl A brief explanation would be highly appreciated + upvotearrow_forwardWhat are the functional groups present in this antibacterial antibiotic? A. Amide, thioether, aldehyde, phenol, carboxylic acid B. Amide, thioether, ketone, amine, phenol, carboxylic acid C. Amide, thioether, ketone, phenol, carboxylic acid D. Thioether, ketone, amine, phenol, carboxylic acid A brief explanation would be highly appreciated + upvotearrow_forwardWhich type of amine is phentermine? a) a primary aliphatic amine b) a primary aromatic amine c) a tertiary aliphatic amine d) a tertiary aromatic aminearrow_forward
- Name each amine. CH;-CH,-N-CH,-CH, b. CH3-CH,-CH2-N-CH3 H CH3 CH3-CH2-CH2-N-CH2-CH;-CH2-CH3arrow_forwardName each amine. b. CH;-CH2-CH2-N-CH, H CH3 c. CH3-CH2-CH;-N-CH,-CH;-CH2-CH;arrow_forwardHave a go at naming amines hello CH, H. CI H H H. H- H H CH,CH,CH, H. H H CH,CH,CH, H CH,CH,CH, Notesarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
