
(a)
Interpretation:
When the given compound is heated, ethene gas is evolved and a product with the formula C14H8O2 is formed. The structure of C14H8O2 is to be drawn from the given data.
Concept introduction:
The Diels-Alder reaction is reversible at high temperature, and this process is called a retro Diels-Alder reaction. The mechanism for this reaction is as the reverse of a Diels-Alder mechanism i.e. the entire six-membered ring is break-down into the corresponding diene and the dienophile. The aromatic proton given signal range from 7 to 9 ppm in 1H NMR spectrum and that is for 13C NMR spectrum the signal range is 120 to 140 ppm.
Answer to Problem 24.82P
The structure of C14H8O2 is
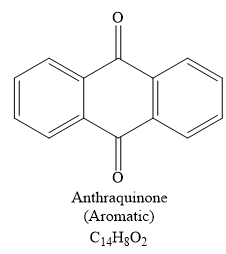
Explanation of Solution
The given compound is
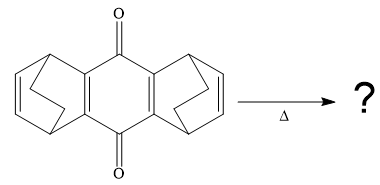
So when this compound is heated, ethene gas is evolved and a product with formula C14H8O2 is formed. Let’s focus on the formula of the product. First, calculate the degree of unsaturation for the product.
DBE =2C - H - X + N + 22
= 2 × 14 - 8 + 22
= 11 (so the compound is aromatic and contains more than one benzene ring)
The signal in 1H NMR the spectrum between 7 and 9 ppm suggests that the compound must be aromatic and more deshielding is due to the presence of carbonyl groups on the benzene ring. The 13C NMR spectrum also gives the signal in the aromatic region and only four signals in 13C NMR spectrum suggest that the product is highly symmetric. Thus the product must be aromatic. The signal at 185 ppm corresponds to the carbonyl carbons. Now focusing on the given compound, which on heating, ethene gas is evolved and the aromatic product with molecular formula C14H8O2 is formed. Thus the retro Diels-Alder reaction may take place as shown below:
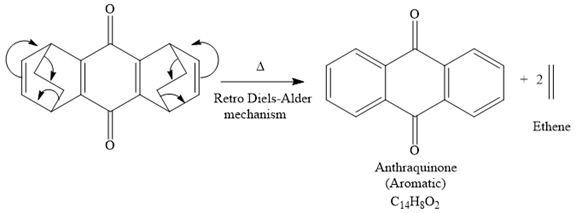
The product of the retro Diels-Alder reaction is also aromatic and matched with the given spectra and also ethene gas is evolved. so the above product is the correct one.
When the given compound is heated, ethene gas is evolved and a product with the formula C14H8O2 is formed. The structure of C14H8O2 is drawn from the given data.
(b)
Interpretation:
When the given compound is heated, ethene gas is evolved and a product with the formula C14H8O2 is formed. The mechanism is to be drawn that accounts for its formation.
Concept introduction:
The Diels-Alder reaction is reversible at high temperature and this process is called a retro Diels-Alder reaction. The mechanism for this reaction is as the reverse of a Diels-Alder mechanism i.e. the entire six-membered ring is broken down into the corresponding diene and the dienophile. The aromatic proton given signal range from 7 to 9 ppm in 1H NMR the spectrum and that is for 13C NMR spectrum the signal range is 120 to 140 ppm.
Answer to Problem 24.82P
The mechanism for the given reaction is
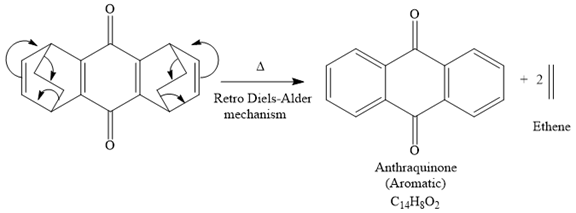
Explanation of Solution
The given compound is

So when this compound is heated, ethene gas is evolved and product with formula C14H8O2 is formed. Let focus on the formula of the product. First, calculate the degree of unsaturation for the product.
DBE =2C - H - X + N + 22
= 2 × 14 - 8 + 22
= 11 (so the compound is aromatic and contains more than one benzene ring)
The signal in 1H NMR spectrum between 7 and 9 ppm suggests that the compound must be aromatic and more deshielding due to the presence of carbonyl groups on the benzene ring. The 13C NMR spectrum also gives the signal in the aromatic region, and the signal at 185 ppm corresponds to the carbonyl carbons, and only four signals in 13C NMR spectrum suggest that the product is highly symmetric. Thus the product must be aromatic. Now focusing on the given compound, which on heating, ethene gas is evolved and the aromatic product with molecular formula C14H8O2 is formed. Thus the retro Diels-Alder reaction may take place as shown below:
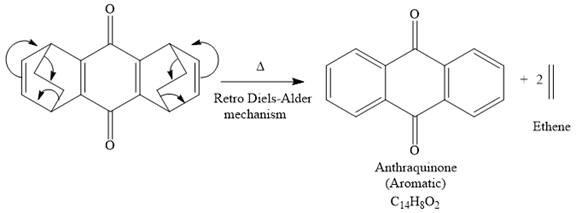
The product of the retro Diels-Alder reaction is also aromatic and matched with the given spectra and also ethene gas is evolved. So the above product and the mechanism (retro Diels-Alder mechanism) is the correct one.
When the given compound is heated, ethene gas is evolved and a product with the formula C14H8O2 is formed. The mechanism is drawn that accounts for its formation.
(c)
Interpretation:
When the given compound is heated, ethene gas is evolved and a product with the formula C14H8O2 is formed. The main driving force that favours the product in the given reaction is to be stated.
Concept introduction:
The Diels-Alder reaction is reversible at high temperature and this process is called a retro Diels-Alder reaction. The mechanism for this reaction is as the reverse of a Diels-Alder mechanism i.e. the entire six-membered ring is break-down into the corresponding diene and the dienophile. The aromatic proton given signal range from 7 to 9 ppm in 1H NMR spectrum and that is for 13C NMR spectrum the signal range is 120 to 140 ppm.
Answer to Problem 24.82P
The main driving force that favours the product in the given reaction is the formation of a more stable aromatic compound.
Explanation of Solution
The given reaction is
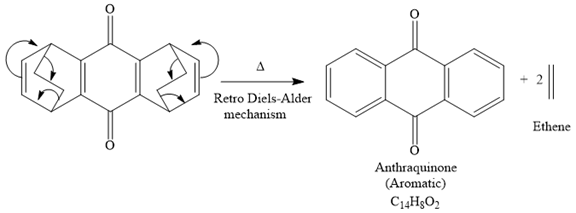
It is noticed that the starting material in the given reaction is overall non-aromatic and also possesses strain due to middle ethylene groups on both sides, but the product is aromatic and strain-free one. Since aromatic compounds are more stable than the non-aromatic compounds this is a key state and the main driving force for the given reaction.
When the given compound is heated, ethene gas is evolved and a product with the formula C14H8O2 is formed. The main driving force that favours the product in the given reaction is stated.
Want to see more full solutions like this?
Chapter 24 Solutions
ORGANIC CHEM PRINC & MECH (BUNDLE)
- Draw the Fischer projection from the skeletal structure shown below. HO OH OH OH OH H Q Drawing Atoms, Bonds and Rings Charges I ☐ T HO H H OH HO I CH2OH H OH Drag H OH -CH2OH CHO -COOH Undo Reset Remove Donearrow_forwardplease provide the structure for this problem, thank youarrow_forwardpresented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forward
- Reaction A 0,0arrow_forwardpresented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward
- 8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forwardpresented by Mr L How the coprion. (Il Done in no wraction, dew the starting redential) доarrow_forward8:16 PM Sun Mar 30 K Draw the major product of this reaction. Ignore inorganic byproducts. Proble 1. CH3MgBr 2. H3O+ F Drawingarrow_forward
- о но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





