
a)
Interpretation:
The relative advantages and disadvantages of packed and open tubular columns in gas chromatography has to be given.
Concept Introduction:
Gas chromatography:
The transportation of gaseous analyte is passed through the column by a gaseous phase known as carrier gas.
Gas-liquid partition chromatography:
A non-volatile liquid is used as stationary phase that is bonded to the inside of the column or to a fine solid support.
Gas-solid adsorption chromatography:
The adsorption of analyte takes place straight on solid particles of the stationary phase.
A general representation of gas chromatograph is shown below,
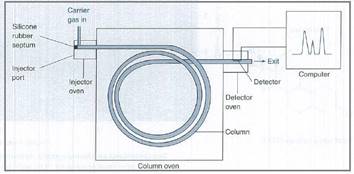
Figure 1
To give the relative advantages and disadvantages of packed and open tubular columns in gas chromatography
b)
Interpretation:
The differences in wall-coated and porous-layer open tubular columns have to be explained.
Concept Introduction:
Open tubular columns:
The open tubular columns are made of silica (in fused form) and is coated with Polyimide for the support and to protect from atmospheric moisture. The most common type of open tubular column is wall coated column and the less common type is porous layer column. The columns are coiled in such way that they fit within a particular temperature controlled column oven.
Advantages and disadvantages of open tubular columns:
Open tubular columns provides higher resolution but short time analysis and show greater sensitivity when compared to packed columns. These columns have less capacity of the sample.
To differentiate wall-coated and porous-layer open tubular columns
c)
Interpretation:
The advantage of bonding the stationary phase to the column wall or cross linking the stationary phase to itself has to be given.
To give the advantage of bonding the stationary phase to the column wall or cross linking the stationary phase to itself
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Quantitative Chemical Analysis
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





