
(a)
Interpretation:
The Fischer projections for the given molecule are to be stated.
Concept introduction:
Fischer projections is a two dimensional representation of organic compounds. It was proposed to represent glucose molecules. The carbon chain is represented vertically; the hydoxy groups and hydrogen atoms are represented horizontally, according to their stereochemistry. The
Answer to Problem 24.1P
The Fischer projections for the given molecule are shown below.
Explanation of Solution
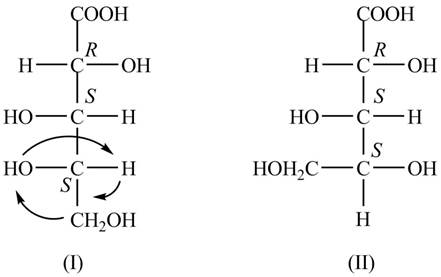
The structure of the given molecule is shown below.
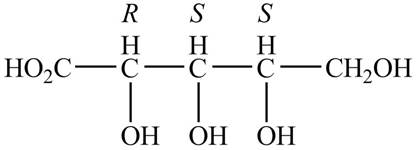
Figure 1
In the Fischer projection, the compound, (2R,3S,4S)-2, 3, 4, 5-tetrahydroxypentanoic acid is represented in two-dimension, the substituent on the carbon chain are placed in such a way that stereochemistry remains same. The groups are interchanged simultaneously such that the stereochemistry of carbon atoms in the second structure also remains same as shown below.
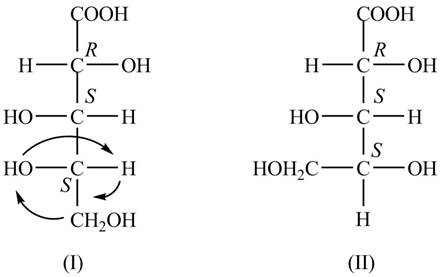
Figure 2
The Fischer projections of (2R,3S,4S)-2, 3, 4, 5-tetrahydroxypentanoic acid are shown in Figure 2.
(b)
Interpretation:
The Fischer projections for (S)-2-butanol are to be stated.
Concept introduction:
Fischer projections is a two dimensional representation of organic compounds. It was proposed to represent glucose molecules. The carbon chain is represented vertically; the hydoxy groups and hydrogen atoms are represented horizontally, according to their stereochemistry. The aldehyde group is paced at the top of the carbon chain and numbered 1 and the ketone group is usually placed at the carbon -2.
Answer to Problem 24.1P
The Fischer projections for (S)-2-butanol are shown below.
Explanation of Solution
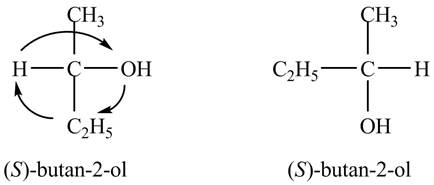
The structure of (S)-2-butanol is shown below.
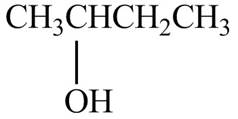
Figure 3
In the Fischer projection, the compound, (S)-2-butanol is represented in two-dimension, the substituent on the carbon chain are placed in such a way that stereochemistry remains same. The groups are interchanged simultaneously in a clockwise direction such that the stereochemistry of carbon atoms in the second structure also remains same as shown below.
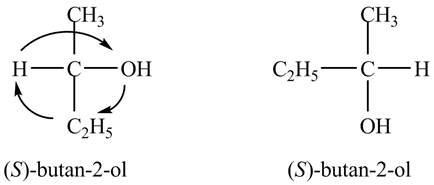
Figure 4
The Fischer projections of (S)-2-butanol are shown in Figure 4.
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry
- Use the References to access important values if needed for this question. What is the IUPAC name of each of the the following? 0 CH3CHCNH₂ CH3 CH3CHCNHCH2CH3 CH3arrow_forwardYou have now performed a liquid-liquid extraction protocol in Experiment 4. In doing so, you manipulated and exploited the acid-base chemistry of one or more of the compounds in your mixture to facilitate their separation into different phases. The key to understanding how liquid- liquid extractions work is by knowing which layer a compound is in, and in what protonation state. The following liquid-liquid extraction is different from the one you performed in Experiment 4, but it uses the same type of logic. Your task is to show how to separate apart Compound A and Compound B. . Complete the following flowchart of a liquid-liquid extraction. Handwritten work is encouraged. • Draw by hand (neatly) only the appropriate organic compound(s) in the boxes. . Specify the reagent(s)/chemicals (name is fine) and concentration as required in Boxes 4 and 5. • Box 7a requires the solvent (name is fine). • Box 7b requires one inorganic compound. • You can neatly complete this assignment by hand and…arrow_forwardb) Elucidate compound D w) mt at 170 nd shows c-1 stretch at 550cm;' The compound has the ff electronic transitions: 0%o* and no a* 1H NMR Spectrum (CDCl3, 400 MHz) 3.5 3.0 2.5 2.0 1.5 1.0 0.5 ppm 13C{H} NMR Spectrum (CDCl3, 100 MHz) Solvent 80 70 60 50 40 30 20 10 0 ppm ppm ¹H-13C me-HSQC Spectrum ppm (CDCl3, 400 MHz) 5 ¹H-¹H COSY Spectrum (CDCl3, 400 MHz) 0.5 10 3.5 3.0 2.5 2.0 1.5 1.0 10 15 20 20 25 30 30 -35 -1.0 1.5 -2.0 -2.5 3.0 -3.5 0.5 ppm 3.5 3.0 2.5 2.0 1.5 1.0 0.5 ppmarrow_forward
- Part I. a) Elucidate the structure of compound A using the following information. • mass spectrum: m+ = 102, m/2=57 312=29 • IR spectrum: 1002.5 % TRANSMITTANCE Ngg 50 40 30 20 90 80 70 60 MICRONS 5 8 9 10 12 13 14 15 16 19 1740 cm M 10 0 4000 3600 3200 2800 2400 2000 1800 1600 13 • CNMR 'H -NMR Peak 8 ppm (H) Integration multiplicity a 1.5 (3H) triplet b 1.3 1.5 (3H) triplet C 2.3 1 (2H) quartet d 4.1 1 (2H) quartet & ppm (c) 10 15 28 60 177 (C=0) b) Elucidate the structure of compound B using the following information 13C/DEPT NMR 150.9 MHz IIL 1400 WAVENUMBERS (CM-1) DEPT-90 DEPT-135 85 80 75 70 65 60 55 50 45 40 35 30 25 20 ppm 1200 1000 800 600 400arrow_forward• Part II. a) Elucidate The structure of compound c w/ molecular formula C10 11202 and the following data below: • IR spectra % TRANSMITTANCE 1002.5 90 80 70 60 50 40 30 20 10 0 4000 3600 3200 2800 2400 2000 1800 1600 • Information from 'HAMR MICRONS 8 9 10 11 14 15 16 19 25 1400 WAVENUMBERS (CM-1) 1200 1000 800 600 400 peak 8 ppm Integration multiplicity a 2.1 1.5 (3H) Singlet b 3.6 1 (2H) singlet с 3.8 1.5 (3H) Singlet d 6.8 1(2H) doublet 7.1 1(2H) doublet Information from 13C-nmR Normal carbon 29ppm Dept 135 Dept -90 + NO peak NO peak 50 ppm 55 ppm + NO peak 114 ppm t 126 ppm No peak NO peak 130 ppm t + 159 ppm No peak NO peak 207 ppm по реак NO peakarrow_forwardCould you redraw these and also explain how to solve them for me pleasarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

