
(a)
Interpretation: Major products for the given set of reactions have to be found.
Concept Introduction:
The reagents such as methyl iodide and silver oxide are given and used as the reaction conditions in the given reactions. This reaction is called Hofmann elimination. The
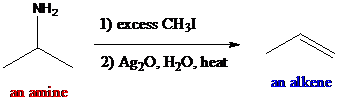
To find: The major product in the reaction (a) via Hofmann elimination
Identify all α- and β-positions in the starting material of the reaction (a)
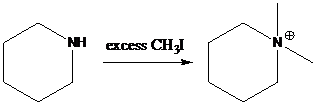
(b)
Interpretation: Major products for the given set of reactions have to be found.
Concept Introduction: The general formula for primary amine is −NH2. There are several methods available to prepare primary amines. Among them, Gabriel synthesis plays a very important role for preparing it. In this method, secondary and tertiary amines are not formed as side products. It involves in three steps.
Formation of potassium phthalimide (deprotonation)
Potassium phthalimide in alkaline KOH acts as the reagent which has negatively charged phthalimide. It is formed by the reaction between phthalimide and potassium hydroxide.
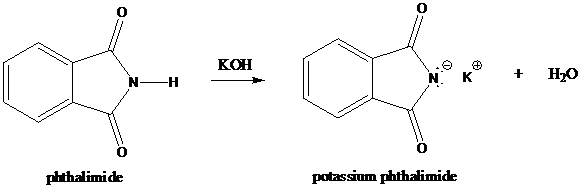
Formation of R−N bond by SN2 nucleophilic substitution
The negative charged nitrogen atom in phthalimide can easily attract the positive side of R−X. In primary
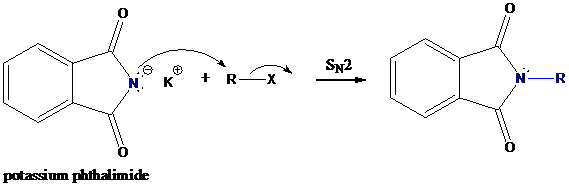
Formation of primary amine by hydrolysis
The resultant product further goes for hydrolysis using hydrazine as the reagent. This reaction also follows nucleophilic substitution reaction. Finally, primary amine is formed with a side product of hydrazine derivative.
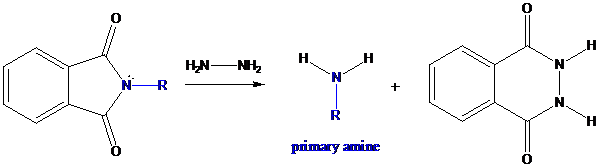
To find: Using a Gabriel synthesis, prepare the major product of (b)
Identify the correct alkyl halide involved in the formation of hexylamine
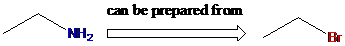
(c)
Interpretation: Major products for the given set of reactions have to be found.
Concept Introduction:
When sodium cyanide is reacted with alkyl halide, SN2 nucleophilic reaction of cyanide on alkyl group occurs. So, cyanide is introduced into alkyl group. By adding water to alkyl cyanide, cyanide is converted into
To find: Obtain the major product in the reaction (c)
Draw the nucleophilic attack of cyanide on alkyl group
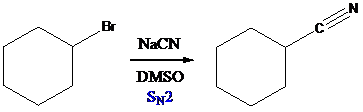
(d)
Interpretation: Major products for the given set of reactions have to be found.
Concept Introduction:
Sandmeyer reactions use copper salts as the reagents. Here, aryldiazonium salt is converted into aryl halides or aryl cyanides by using copper halides or copper cyanides.
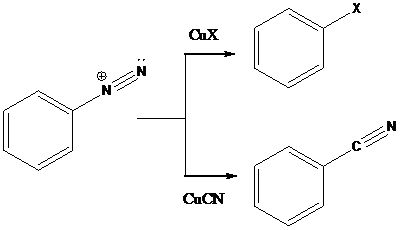
To find: The major product in the reaction (d) via Sandmeyer reaction
Do nitration of benzene

Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





