
Concept explainers
(a)
Interpretation: The products formed by the reaction of
Concept introduction: Nucleophilic addition reaction is a type of organic reaction in which the nucleophile is added to the electrophilic site. The carbon atom in carbonyl compound acts as an electrophilic centre where a nucleophile attacks and gives an addition product.
Answer to Problem 23.49P
The product formed by the reaction of
Explanation of Solution
The given compound is,
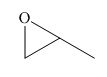
Figure 1
The nucleophile
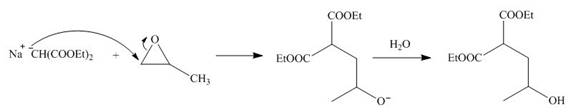
Figure 2
On the further treatment of
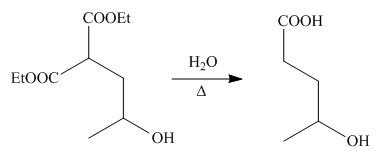
Figure 3
Hence, the product formed by the reaction of
The product formed by the reaction of
(b)
Interpretation: The products formed by the reaction of
Concept introduction: Nucleophilic addition reaction is a type of organic reaction in which the nucleophile is added to the electrophilic site. The carbon atom in carbonyl compound acts as an electrophilic centre where a nucleophile attacks and gives an addition product.
Answer to Problem 23.49P
The product formed by the reaction of
Explanation of Solution
The given compound is,
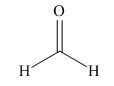
Figure 4
The nucleophile
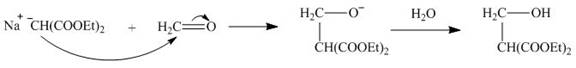
Figure 5
On the further treatment of
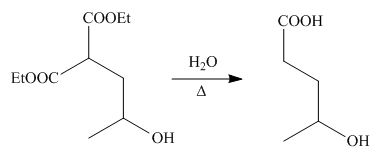
Figure 6
Hence, the product formed by the reaction of
The product formed by the reaction of
(c)
Interpretation: The products formed by the reaction of
Concept introduction: Nucleophilic addition reaction is a type of organic reaction in which the nucleophile is added to the electrophilic site. The carbon atom in carbonyl compound acts as an electrophilic centre where a nucleophile attacks and gives an addition product.
Answer to Problem 23.49P
The product formed by the reaction of
Explanation of Solution
The given compound is,
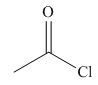
Figure 7
The nucleophile
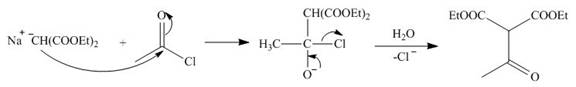
Figure 8
On the further treatment of
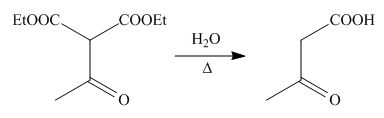
Figure 9
Hence, the product formed by the reaction of
The product formed by the reaction of
(d)
Interpretation: The product formed by the reaction of
Concept introduction: Nucleophilic addition reaction is a type of organic reaction in which the nucleophile is added to the electrophilic site. The carbon atom in carbonyl compound acts as an electrophilic centre where a nucleophile attacks and gives an addition product.
Answer to Problem 23.49P
The products formed by the reaction of
Explanation of Solution
The given compound is,
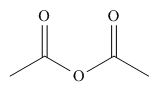
Figure 10
The nucleophile
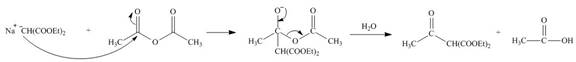
Figure 11
On the further treatment of
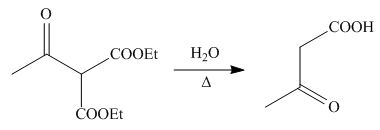
Figure 12
Hence, the products formed by the reaction of
The products formed by the reaction of
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry-Package(Custom)
- What explains why many aldehydes and ketones can undergo self-condensation reactions in basic conditions? The alpha carbon can lose a proton and act like a nucleophile and the carbonyl carbon is an electrophile. The alpha carbon can gain a proton and act like an electrophile and the carbonyl carbon is a nucleophile. The oxygen of the carbonyl group can attack the carbon of the carbonyl group. Only esters can undergo self-condensation reactions.arrow_forwardIdentify the best reagents to complete the following reaction.arrow_forwardIdentify the best reagents to complete the following reaction. HO, CIarrow_forward
- Draw the structure of the major organic product(s) of the reaction. H3O +arrow_forwardExplain the relevance pi* (antibonding) -molecular orbitals have in nucleophilic addition reactions of carbonyl containing compounds.arrow_forwardOH 6 From ethyl bromide and a substituted phenyl derivativearrow_forward
- Draw the products formed when each compound is treated with HNO3 and H2SO4.State whether the reaction occurs faster or slower than a similar reaction with benzene.arrow_forwardDraw the structures of the initially formed enol tautomers in the reactions of propyne and dicyclohexylethyne with dicyclohexylborane followed by NaOHNaOH and H2O2H2O2arrow_forwardIdentify the best reagents to complete the following reaction. Options are included.arrow_forward
- What explains why many aldehydes and ketones can undergo self- condensation reactions in basic conditions? The alpha carbon can lose a proton and act like a nucleophile and the carbonyl carbon a an electrophile O The alpha carbon can gain a proton and act like an electrophile and the carbonyl carbon is a nucleophile O The oxygen of the carbonyl group can attack the carbon of the carbonyl group Only esters can undergo self-condensation reactionsarrow_forwardAcetylene reacts with sodium amide in the presence of propyl halide produces aldehyde produces ketones It produces 2-pentanearrow_forwardWhen propene reacts with gaseous hydrogen bromide, HBr, two products, 1-bromopropane and 2-bromopropane are formed. The reaction is a two-step process in which the electrophilic attack occurs in the first step. Identify the electrophile in this reaction Draw a diagram showing the first step of the reaction that leads to the production of 2-bromopropane.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





