
(a)
Interpretation: To classify each of the following molecules as (1) an oxidizing agent, (2) a reducing agent, or (3) neither an oxidizing agent nor a reducing agent.
a. NADH
b. ATP
c. FAD
d. CoA–SH
Concept introduction: The sum of various
ATP is a
Flavin adenine dinucleotideexists in two forms: oxidized form
Coenzyme A (CoA) is a coenzyme which is utilized in various metabolic reactions. The functions of coenzyme A include oxidation of pyruvate in the citric cycle and fatty acid oxidation.
Oxidizing agents are those species which gets reduced and oxidizes the other species present in the chemical reaction. Reducing agent is those species which gets oxidized and reduces the other species present in a chemical reaction. Generally, oxidizing agents are electron acceptor and reducing agents are electron donor.
(a)
Answer to Problem 23.44EP
Explanation of Solution
Nicotinamide adenine dinucleotide exists in two forms:
Here
(b)
Interpretation: To classify each of the following molecules as (1) an oxidizing agent, (2) a reducing agent, or (3) neither an oxidizing agent nor a reducing agent.
a. NADH
b. ATP
c. FAD
d. CoA–SH
Concept introduction: The sum of various chemical reactions occurring in the human body is called metabolism and the reactions individually are known as metabolic reactions. During these metabolic reactions, the various metabolic intermediates are formed for the short time to complete the reactions.ATP,
ATP is a nucleotide which provides energy for the completion of various metabolic reactions occurring in our human body. The structure of ATP consists of adenine base, ribose sugar unit and the three phosphate groupconnected to each other by phosphoanhydride bonds.
Flavin adenine dinucleotideexists in two forms: oxidized form
Coenzyme A (CoA) is a coenzyme which is utilized in various metabolic reactions. The functions of coenzyme A include oxidation of pyruvate in the citric cycle and fatty acid oxidation.
Oxidizing agents are those species which gets reduced and oxidizes the other species present in the chemical reaction. Reducing agent is those species which gets oxidized and reduces the other species present in a chemical reaction. Generally, oxidizing agents are electron acceptor and reducing agents are electron donor.
(b)
Answer to Problem 23.44EP
ATP molecule is neither a reducing agent nor an oxidizing agent in metabolic reactions.
Explanation of Solution
Adenosine triphosphate (ATP) is anucleotide which structural component is one unit of the adenine base, one unit of ribose sugar and three units of a phosphate group. It can be converted into its monophosphate form(AMP) and diphosphate form(ADP) by losing a phosphate group. The reaction to this change is:
Here ATP is not involved in electron transfer hence it is neither a reducing agent nor an oxidizing agent.
(c)
Interpretation: To classify each of the following molecules as (1) an oxidizing agent, (2) a reducing agent, or (3) neither an oxidizing agent nor a reducing agent.
a. NADH
b. ATP
c. FAD
d. CoA–SH
Concept introduction: The sum of various chemical reactions occurring in the human body is called metabolism and the reactions individually are known as metabolic reactions. During these metabolic reactions, the various metabolic intermediates are formed for the short time to complete the reactions.ATP,
ATP is a nucleotide which provides energy for the completion of various metabolic reactions occurring in our human body. The structure of ATP consists of adenine base, ribose sugar unit and the three phosphate groupconnected to each other by phosphoanhydride bonds.
Flavin adenine dinucleotideexists in two forms: oxidized form
Coenzyme A (CoA) is a coenzyme which is utilized in various metabolic reactions. The functions of coenzyme A include oxidation of pyruvate in the citric cycle and fatty acid oxidation.
Oxidizing agents are those species which gets reduced and oxidizes the other species present in the chemical reaction. Reducing agent is those species which gets oxidized and reduces the other species present in a chemical reaction. Generally, oxidizing agents are electron acceptor and reducing agents are electron donor.
(c)
Answer to Problem 23.44EP
Explanation of Solution
Flavin adenine dinucleotide exists in two forms:
Here
(d)
Interpretation: To classify each of the following molecules as (1) an oxidizing agent, (2) a reducing agent, or (3) neither an oxidizing agent nor a reducing agent.
a. NADH
b. ATP
c. FAD
d. CoA–SH
Concept introduction: The sum of various chemical reactions occurring in the human body is called metabolism and the reactions individually are known as metabolic reactions. During these metabolic reactions, the various metabolic intermediates are formed for the short time to complete the reactions.ATP,
ATP is a nucleotide which provides energy for the completion of various metabolic reactions occurring in our human body. The structure of ATP consists of adenine base, ribose sugar unit and the three phosphate groupconnected to each other by phosphoanhydride bonds.
Flavin adenine dinucleotideexists in two forms: oxidized form
Coenzyme A (CoA) is a coenzyme which is utilized in various metabolic reactions. The functions of coenzyme A include oxidation of pyruvate in the citric cycle and fatty acid oxidation.
Oxidizing agents are those species which gets reduced and oxidizes the other species present in the chemical reaction. Reducing agent is those species which gets oxidized and reduces the other species present in a chemical reaction. Generally, oxidizing agents are electron acceptor and reducing agents are electron donor.
(d)
Answer to Problem 23.44EP
Coenzyme A (CoA–SH) molecule is neither a reducing agent nor an oxidizing agent in metabolic reactions.
Explanation of Solution
Coenzyme A (CoA) is a coenzyme whose structure is based on the B vitamin pantothenic acid. Its structure consists of three subunits: 2-Aminoethanethiol, pantothenic acid, and phosphorylated ADP.
Coenzyme A is always in equilibrium with its acetyl form and therefore helps in transfer of acetyl group in metabolic reaction. The reaction for this change is
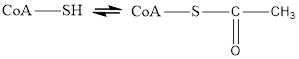
Here Coenzyme A (CoA) is not involved in electron transfer hence it is neithera reducing agent nor an oxidizing agent.
a.
b. ATP molecule is neither a reducing agent nor an oxidizing agent in metabolic reactions.
c.
d. Coenzyme A (CoA–SH) molecule is neither a reducing agent nor an oxidizing agent in metabolic reactions.
Want to see more full solutions like this?
Chapter 23 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- Which of the following statements is TRUE? Select one: A. Lactic acid is a product of aerobic respiration; ethyl alcohol is a product of fermentation. B. Oxidation is the loss of electrons; reduction is the gain of electrons. C. Oxygen is a product of cellular respiration; carbon dioxide is a product of photosynthesis. D. Glucose is a product of aerobic respiration; lactic acid is a product of anaerobic respiration.arrow_forwardWhat would the net amount of ATP produced be if dehydrogenase was inhibited for 2 molecules of glucose? a.) 0 b.) 2 c.) 4 d.) -2 e.) -4arrow_forwardRapidly dividing cells such as bone marrow, skin, intestinal mucosa, and cancer cells need DNA synthesis. In these cells, the following is observed: a. a decreased NADPH / NADP+ ratio b. increased flux through the oxidative reactions c. Flux through the oxidative reactions is low and the nonoxidative reactions are reversed to make ribose 5-phosphate. d. Ribose 5-phosphate is recycled through the oxidative steps via the nonoxidative reactions and gluconeogenesis. e. Ribose 5-phosphate is shunted into glycolysis by the nonoxidative reactions.arrow_forward
- Which of the following statements concerning ATP is true? a. The free energy value for the hydrolysis of ATP is nearly the same for ADP. b. The free energy value for the hydrolysis of ATP is greater than that for ADP. c. ATP hydrolysis is more likely at pH 5 than at pH 7. d. One mole of glycerate-1,3-bisphosphate can phosphorylate one mole of AMP to yield ATP.arrow_forwardWhich of the following is NOT a role of coenzymes? A. Receiver or donor of hydrides in redox reactions B. Receiver or donor of carboxyl groups in reactions that involve carboxyl group transfer. C. Receiver or donor of amino groups in transamination and deamination reactions. D. Receiver or donor of alkyl, alkenyl, acyl, or formyl groups in transfer reactions. E. Receiver or donor of oxygen in oxygenation reactions.arrow_forwardDefine the following terms: a. Fe–S clusters b. electron transport c. CoQ d. ubisemiquinone e. UQH2arrow_forward
- ATP inhibits phosphofructokinase by binding to an allosteric site in glycolysis. ATP is functioning as a a. competitive inhibitor. b. competitive activator. c. noncompetitive inhibitor. d. noncompetitive activator.arrow_forwardWhich of the following compounds would you expect to liberate the least free energy when hydrolyzed? Explain. a. ATP b. ADP c. AMP d. phosphoenolpyruvate e. phosphocreatinearrow_forwarda. What is the name of metabolite 1? b. What enzyme converts metabolite 1 to metabolite 2 (E1)? c. What is the name of metabolite 2? d. What enzyme converts metabolite 4 to metabolite 5 (E4)? e. What cofactor, if any, would E4 require?arrow_forward
- Chemotrophic energy metabolism is a catabolic and exergonic process that produces ATP from oxidizing molecules. Which among the following is not an energy source of chemotrophic energy metabolism? A. Iron B. Photons C. Carbohydrates D. Proteinsarrow_forwardList the following substances in order of increasing tendency to accept e-: a. pyruvate, b. CoQ, c. O2 d. FADarrow_forwardThe oxidative phase of the pentose phosphate pathway produces which of the following (note - select all that apply)? A. ATP B. Glucose-6-phosphate C. NADPH D. Ribose-5-phosphatearrow_forward
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education





