
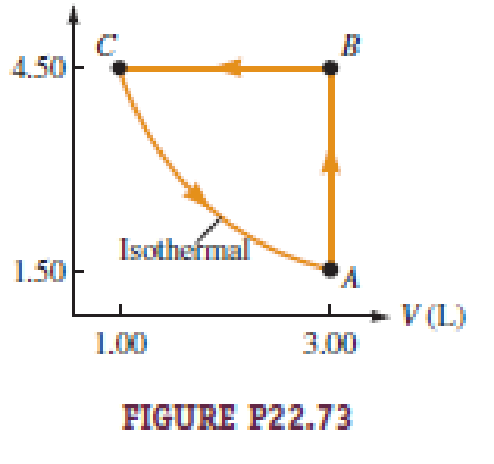
Figure P22.73 illustrates the cycle ABCA for a 2.00-mol sample of an ideal diatomic gas, where the process CA is a reversible isothermal expansion. What is a. the net work done by the gas during one cycle? b. How much energy is added to the gas by heat during one cycle? c. How much energy is exhausted from the gas by heat during one cycle? d. What is the efficiency of the cycle? e. What would be the efficiency of a Carnot engine operated between the temperatures at points A and B during each cycle?
(a)
The net work done by the gas during one cycle.
Answer to Problem 73PQ
The net work done by the gas during one cycle is
Explanation of Solution
The work done by the gas along AB is
Write the expression to calculate the work done by the gas along BC.
Here,
Write the expression to calculate the work done by the gas along CA.
Here,
Write the expression to calculate the total work done by the gas.
Here, W is the total work done by the gas.
Conclusion:
Substitute
Substitute
Substitute
Therefore, the net work done by the gas during one cycle is
(b)
The heat added in one cycle.
Answer to Problem 73PQ
The heat added in one cycle is
Explanation of Solution
For the diatomic gases, the specific heat capacity at constant volume is
The temperature at A and C of the gas is same.
Write the expression to calculate the temperature of the gas at A and C.
Here, T is the temperature of the gas at A and C, P is the pressure at A, V is the pressure at A n is the number of moles and R is the universal gas constant.
Write the expression to calculate the temperature at B.
Here,
Write the expression to calculate the energy added to the system.
Here, Q is the energy added to the system,
Substitute
Conclusion:
Substitute
Substitute
Substitute
Therefore, the heat added in one cycle is
(c)
The energy exhausted from the gas.
Answer to Problem 73PQ
The energy exhausted from the gas is
Explanation of Solution
For the diatomic gases, the specific heat capacity at constant pressure is
Write the expression to calculate the heat exhausted from the gas.
Here,
Substitute
Conclusion:
Substitute
Therefore, the energy exhausted from the gas is
(d)
The efficiency of the cycle.
Answer to Problem 73PQ
The efficiency of the cycle is
Explanation of Solution
Write the expression to calculate the efficiency of one cycle.
Here, e is the efficiency.
Conclusion:
Substitute
Therefore, the efficiency of the cycle is
(e)
The efficiency of the Carnot engine.
Answer to Problem 73PQ
The efficiency of the Carnot engine is
Explanation of Solution
Write the expression to calculate the efficiency of the Carnot engine.
Here,
Conclusion:
Substitute
Therefore, the efficiency of the Carnot engine is
Want to see more full solutions like this?
Chapter 22 Solutions
Webassign Printed Access Card For Katz's Physics For Scientists And Engineers: Foundations And Connections, 1st Edition, Single-term
- A thermodynamic cycle is shown in Figure P21.34 for a gas in a piston. The system changes states along the path ABCA. a. What is the total work done by the gas during this cycle? b. How much heat is transferred? Does heat flow into or out of the system? Figure P21.34arrow_forward(a) In reaching equilibrium, how much heat transfer occurs from 1.00 kg of water at 40.0C when it is placed in contact with 1.00 kg of 20.0C water in reaching equilibrium? (b) What is the change in entropy due to this heat transfer? (c) How much work is made unavailable, taking the lowest temperature to be 20.0C ? Explicitly show how you follow the steps in the Problem-Solving Strategies for Entropy.arrow_forwardFigure P21.36 shows a cyclic thermodynamic process ABCA for an ideal gas. a. What is the net energy transferred into the system by heat during each cycle? b. What would be the net energy transferred into the system by heat if the cycle followed the path ACBA instead? FIGURE P21.36 FIGURE P21.37arrow_forward
- (a) What is the best coefficient of performance for a heat pump that has a hot reservoir temperature of 50.0C and a cold reservoir temperature of 20.0C ? (b) How much heat transfer occurs into the warm environment if 3.60107J of work (10.0kWh) is put into it? (c) If the cost of this work input is 10.0cent/kWh, haw does its cost compare with the direct heat transfer achieved by burning natural gas at a cost of 85.0 cents per therm. (A therm is a common unit of energy for natural gas and equals 1.055108J .)arrow_forwardA power plant has been proposed that would make use of the temperature gradient in the ocean. The system is to operate between 20.0C (surface water temperature) and 5.00C (water temperature at a depth of about 1 km). (a) What is the maximum efficiency of such a system? (b) If the useful power output of the plant is 75.0 MW, how much energy is absorbed per hour? (c) In view of your answer to part (a), do you think such a system is worthwhile (considering that there is no charge for fuel)?arrow_forward(a) What is the best coefficient of performance for a refrigerator that cools an environment at 30.0C and has heat transfer to another environment at 45.0C ? (b) How much work in joules must be done for a heat transfer of 4186 kJ from the cold environment? (c) What is the cost of doing this if the work costs 10.0 cents per 3.60106J (a kilowatthour)? (d) How many kJ of heat transfer occurs into the warm environment? (e) Discuss what type of refrigerator might operate between these temperatures.arrow_forward
- (a) What is the change in entropy if you start with 100 coins in the 45 heads and 55 tails macrostate, toss them, and get 51 heads and 49 tails? (b) What if you get 75 heads and 25 tails? (c) How much more likely is 51 heads and 49 tails than 75 heads and 25 tails? (d) Dues either outcome violate the second law of thermodynamics?arrow_forward(a) How much heat transfer occurs from 20.0 kg of 90.0C water placed in contact with 20.0 kg of 10.0C water, producing a final temperature of 50.0C ? (b) How much work could a Carnot engine do with this heat transfer, assuming it operates between two reservoirs at constant temperatures of 90.0C and 10.0C ? (c) What increase in entropy is produced by mixing 20.0 kg of 90.0C water with 20.0 kg of 10.0C water? (d) Calculate the amount of work made unavailable by this mixing using a low temperature of 10.0C, and compare it with the work done by the Garnet engine. Explicitly show how you follow the steps in the Problem-Solving Strategies for Entropy. (e) Discuss how everyday processes make increasingly more energy unavailable to do work, as implied by this problem.arrow_forwardA sample of a monatomic ideal gas is contained in a cylinder with a piston. Its stale is represented by the dot in the PV diagram shown in Figure OQ22.9. Arrows A through E represent isobaric, isothermal, adiabatic, and isovolumetric processes that the sample can undergo. In each process except D, the volume changes by a factor of 2. All five processes are reversible. Rank the processes according to the change in entropy of the gas from the largest positive value to the largest-magnitude negative value. In your rankings, display any cases of equality.arrow_forward
- An ideal gas with specific heat ratio confined to a cylinder is put through a closed cycle. Initially, the gas is at Pi, Vi, and Ti. First, its pressure is tripled under constant volume. It then expands adiabatically to its original pressure and finally is compressed isobarically to its original volume. (a) Draw a PV diagram of this cycle. (b) Determine the volume at the end of the adiabatic expansion. Find (c) the temperature of the gas at the start of the adiabatic expansion and (d) the temperature at the end of the cycle. (e) What was the net work done on the gas for this cycle?arrow_forwardP (Pa) 800 B 600 400 A C 200 0 2 8 10 v (m³) 4 6 4. One mole of an ideal gas is taken through a closed cycle A→B→C→A. Use the information from the graph to answer the following questions: a. Find the temperature at point A. b.ls the net work done by the gas positive or negative? Explain. c. How much work is done by the gas? d.ls heat added to the gas or removed from the gas for the entire cycle? Explain. e. How much heat is added to the gas? f. What is the change in internal energy for the entire cycle? Answers:arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





