
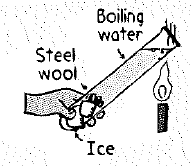
Hold the bottom end of a test tube full of cold water in your hand. Heat the top part in a flame until the water boils. The fact that you can still hold the bottom shows that water is a poor conductor of heat. This is even more dramatic when you wedge chunks of ice at the bottom; then the, water above can be brought to a boil without melting the ice. Try it and see.
Relation between temperature and thermal energy of an object
Explanation of Solution
Introduction:
Definition of thermal energy:
Thermal energy, also referred to as heat, is produced when an increase in temperature causes atoms and molecules to maneuver faster and hit one another. The energy that comes from the temperature of the heated substance is named thermal energy.
Definition of temperature:
Temperature is the measurement of hotness or coldness of a body. It is expressed in terms of any of various arbitrary scales and it also indicates the direction during which heat will spontaneously flow i.e., from a warmer body (one at a better temperature) to a colder body (one at a lower temperature).
Heat is that the one sort of energy which is in transition condition due to its temperature difference and temperature is that the measure of warmth.
We have all noticed that once we heat something up, its temperature rises. Often we expect that heat and temperature are an equivalent thing. However, this is often not the case. Heat and temperature are associated with one another, but are different concepts.
Heat is that the total energy of molecular motion during a substance while temperature may be a measure of the typical energy of molecular motion during a substance. Heat depends on the speed of the particles, the amount of particles, the dimensions or mass of particles, and therefore the sort of particles in an object but temperature doesn't depend upon the dimensions or sort of object.
For example, the temperature of a little cup of water could be an equivalent because the temperature of an outsized tub of water, but the bathtub of water has more heat because it's more water and thus more total thermal energy.
Temperature is often positive or negative but thermal energy always has positive values.
Conclusion:
Temperature doesn't depend upon the number of the substance. It’s associated with the typical K.E. of the particles. On the opposite hand the thermal energy depends on the number of the substances. It’s associated with the entire K.E. of the particles.
Chapter 22 Solutions
Conceptual Physics: The High School Physics Program
Additional Science Textbook Solutions
University Physics with Modern Physics (14th Edition)
The Cosmic Perspective Fundamentals (2nd Edition)
Conceptual Integrated Science
Physics for Scientists and Engineers: A Strategic Approach with Modern Physics (4th Edition)
Essential University Physics: Volume 2 (3rd Edition)
Conceptual Physics (12th Edition)
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





