
Chemistry: A Molecular Approach (4th Edition)
4th Edition
ISBN: 9780134112831
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 22, Problem 34E
Interpretation Introduction
Interpretation: The following fatty acid is most likely to be a solid at room temperature or not.
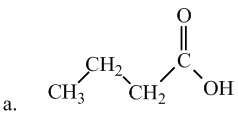
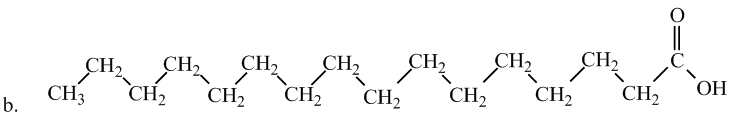
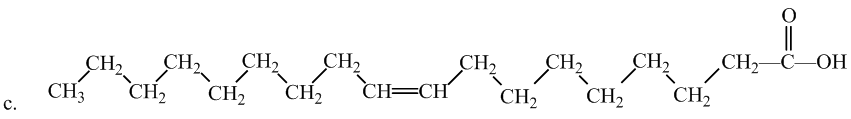
Concept Introduction:
Saturated fatty acids experience greater molecular forces, making them solid at room temperature. Therefore, option (b) is most likely to be a solid at room temperature. The hydrocarbon chains in this fatty acid are fairly straight and packed closely together, making it solid at room temperature.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Review: Design a total
total
synthesis
synthesis
of the following compound using
methyloxacyclopropane and any other necessary reagents.
None
What 3 steps could you use to make the following product from the giving starting
material?
HO
Chapter 22 Solutions
Chemistry: A Molecular Approach (4th Edition)
Ch. 22 - Prob. 1SAQCh. 22 - Prob. 2SAQCh. 22 - Prob. 3SAQCh. 22 - Prob. 4SAQCh. 22 - Prob. 5SAQCh. 22 - Prob. 6SAQCh. 22 - Prob. 7SAQCh. 22 - Prob. 8SAQCh. 22 - Q9. Peptide bonds occur in which type of...Ch. 22 - Q10. How many nucleotides are required to code for...
Ch. 22 - 1. What is biochemistry? What significant advances...Ch. 22 - Prob. 2ECh. 22 - Prob. 3ECh. 22 - 4. What effect do double bonds have within the...Ch. 22 - Prob. 5ECh. 22 - Prob. 6ECh. 22 - 7. Describe the basic structure of phospholipids...Ch. 22 - 8. What is a steroid? List some functions of...Ch. 22 - Prob. 9ECh. 22 - Prob. 10ECh. 22 - Prob. 11ECh. 22 - Prob. 12ECh. 22 - Prob. 13ECh. 22 - Prob. 14ECh. 22 - Prob. 15ECh. 22 - Prob. 16ECh. 22 - Prob. 17ECh. 22 - Prob. 18ECh. 22 - Prob. 19ECh. 22 - Prob. 20ECh. 22 - Prob. 21ECh. 22 - Prob. 22ECh. 22 - Prob. 23ECh. 22 - Prob. 24ECh. 22 - Prob. 25ECh. 22 - Prob. 26ECh. 22 - 27. What is a codon? A gene? A chromosome?
Ch. 22 - Prob. 28ECh. 22 - Prob. 29ECh. 22 - Prob. 30ECh. 22 - Prob. 31ECh. 22 - Prob. 32ECh. 22 - Prob. 33ECh. 22 - Prob. 34ECh. 22 - 35. Draw structures showing the reaction of...Ch. 22 - Prob. 36ECh. 22 - Prob. 37ECh. 22 - Prob. 38ECh. 22 - Prob. 39ECh. 22 - Prob. 40ECh. 22 - Prob. 41ECh. 22 - Prob. 42ECh. 22 - Prob. 43ECh. 22 - Prob. 44ECh. 22 - Prob. 45ECh. 22 - Prob. 46ECh. 22 - Prob. 47ECh. 22 - Prob. 48ECh. 22 - 49. Draw each amino acid in its dipolar ion...Ch. 22 - 50. Draw each amino acid in its dipolar ion...Ch. 22 - Prob. 51ECh. 22 - Prob. 52ECh. 22 - Prob. 53ECh. 22 - Prob. 54ECh. 22 - Prob. 55ECh. 22 - Prob. 56ECh. 22 - Prob. 57ECh. 22 - Prob. 58ECh. 22 - 59. A phenylalanine amino acid on a protein strand...Ch. 22 - 60. An amino acid on a protein strand forms a...Ch. 22 -
61. The amino acid sequence in one section of a...Ch. 22 - Prob. 62ECh. 22 - Prob. 63ECh. 22 - Prob. 64ECh. 22 - Prob. 65ECh. 22 - Prob. 66ECh. 22 - Prob. 67ECh. 22 - Prob. 68ECh. 22 - Prob. 69ECh. 22 - Prob. 70ECh. 22 - 71. Determine the class of biochemical compound...Ch. 22 - Prob. 72ECh. 22 - 73. What is the difference between a codon and a...Ch. 22 - 74. What is the difference between a fatty acid...Ch. 22 - Prob. 75ECh. 22 - Prob. 76ECh. 22 - Prob. 77ECh. 22 - Prob. 78ECh. 22 - 79. Determining the amino acid sequence in a...Ch. 22 - Prob. 80ECh. 22 - Prob. 81ECh. 22 - 82. Calculate the mass percent of phosphorus in a...Ch. 22 - 83. The double helical structure of DNA disrupts...Ch. 22 - Prob. 84ECh. 22 - Prob. 85ECh. 22 - Prob. 86ECh. 22 - Prob. 87ECh. 22 - Prob. 88ECh. 22 - 89. Write the major equilibrium that is...Ch. 22 - Prob. 90ECh. 22 - Prob. 91ECh. 22 - 92. The genetic code is random, which means that a...Ch. 22 - Prob. 93QGWCh. 22 - Prob. 94QGWCh. 22 - Prob. 95QGWCh. 22 - Prob. 96QGWCh. 22 - Prob. 97QGWCh. 22 - Prob. 98DIA
Knowledge Booster
Similar questions
- Which of the following would you expect to be antiaromatic? Please provide a detailed explanation.arrow_forwardNonearrow_forwardDraw a Newman projection from carbon 3 to carbon 2 in the highest energy conformation for the following molecule. What is this conformation called? What kind of strain is present? Brarrow_forward
- Which of the following dienophiles is most reactive in a Diels-Alder reaction: Please explain why the correct answer to this question is option 5. Please provide a detailed explanation.arrow_forwardWhich of the following would you expect to be aromatic? Please provide a detailed explanation.arrow_forwardDraw the enantiomer and diastereomers of the following molecule. Label each type of stereoisomers. Label each chiral center as R or S. HOarrow_forward
- Which diene and dienophile would you choose to synthesize the following compound? Please provide a detailed explanation. Please include a drawing showing the mechanism of the synthesis. Please also explain why it is the correct diene and dienophile.arrow_forwardUsing the sketcher below, draw the structure of N-ethyldecylamine. Answer: 0 ୨୫) . 始 {n [ ]t ?arrow_forwardWhich of the following would you expect to be aromatic? Please provide a detailed explanation.arrow_forward
- Identify the characteristic signals that you would expect in the diagnostic region of an IR spectrum of each of the following compounds. a. H₂N b.arrow_forwardWhat is the lowest energy chair for the following cyclohexane? ' || || a. b. " " d.arrow_forwardAnswer the following questions using the below figure: Potential Energy ри Reaction Progress a. How many transition states occur in this reaction? b. How many intermediates occur in this reaction? c. Is this reaction spontaneous or nonspontaneous? d. Does this reaction have a positive or negative AG? e. Label the activation energy(ies).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY