
Interpretation:
The structural formulas for all the
Concept introduction:
If the molecular formula of two compounds is same but their atoms are connected in different ways, they are known as constitutional or structural isomers.
In primary amines, the nitrogen atom is connected to two hydrogen atoms. In secondary amines, the nitrogen atom is connected to one hydrogen atom and tertiary amines have no hydrogen atom connected to the nitrogen atom.
Answer to Problem 23P
Solution:
The structural formulas for all the amines of molecular formula
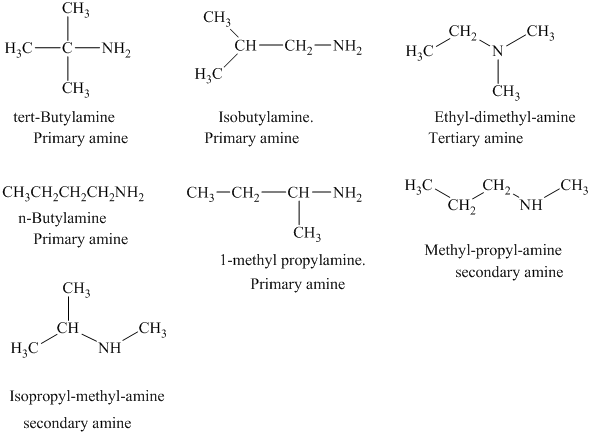
Explanation of Solution
The given molecular formula is

In each of the above isomer, the
The structural formulas for all the seven isomeric amines possible for the molecular formula
Want to see more full solutions like this?
Chapter 22 Solutions
ORGANIC CHEMISTRY-W/STUD.SOLN.MAN.
- Draw the structures of the amines from which phenylephrine hydrochloride and methadone hydrochloride are derived.arrow_forwardFexofenadine is a non-sedating antihistamine with a single amine functional group. Classify the amine in fexofenadine. 2⁰ 4º 1⁰ 3⁰ OH OH OHarrow_forwardDraw structural formulas for all the amines having the formula C3H9N. Classify each as either a primary amine, a secondary amine, or a tertiary amine.arrow_forward
- < app.101edu.co Classify and describe the properties of the following nitrogen containing compound. O CI O + Question 13.a of 25 CH3 N Classify the following amine. H CH3 A) primary amine B) secondary amine C) tertiary amine @=J D) tertiary amine salt E) quaternary ammonium saltarrow_forwardComplete this table for different amine compounds. Chemical propylamine quaternary ammonium ion methylphenylamine Molecular formula C3H9N C₂H7N C5H13N C4H10N Structural formula (CH3)3N (CH3)2NH CH 3 I (CH₂) 11 | H3C(CH2) 11 -N-(CH₂)11CH3 NHCH3 (CH₂)11 CH3 CH3CH2CH2-NH-CH3 + Classification Tertiary Quaternary Tertiary Secondaryarrow_forwardWrite the common name for each amine. HỌC—N—CH,CH3 H₂C-CH₂-CH₂-CH₂ Ethylbutylamine common name: Incorrect HC—CH,—N—CH,—CHy CH₂ CH3 diethylethylamine common name: Incorrect These compounds are tertiary amines.arrow_forward
- Compare the table below by giving the structure and name of a primary (10), secondary(20), and tertiary(30) amine with molecular formula C3H9N. Primary amines can be prepared from amides by Hoffman's reaction. Write a general equation for the reaction and give reagents with reaction conditions for this reaction.arrow_forwardDraw structural formulas for the three secondary amines with the molecular formula C4H11N.arrow_forwardAmides undergo hydrolysis under acidic conditions to give Select one: O Carboxylate ion and ammonium ion Carboxylic acid and amine Carboxylate ion and amine Carboxylic acid and ammonium ionarrow_forward
- Explain why CH3 CH2 CH2NH2 is a Brønsted base. Its water solutions are basic. All substances containing nitrogen atoms are Brønsted bases. This amine is a proton donor. This amine can ассеpt a proton from a proton donor.arrow_forwardThree amide isomers, N,N-dimethylformamide, N-methylacetamide, and propanamide, have respective boiling points of 153 °C (426 K), 202 °C (475 K), and 213 °C (486 K). Explain these boiling points in light of their structural formulas.arrow_forwardThe structure of Amines are classified as primary (1), secondary (2), and tertiary (3) that concept that seemed difficult to you at first, but then after working on the concept, you were able to master it. Include a description of what made the concept difficult at first, and then discuss what you did in order to better understand the concept.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,



