
(a)
Interpretation:
The enol structure of the compound
Concept introduction:
Answer to Problem 22.7P
The enol structures of the compound
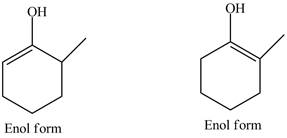
Explanation of Solution
The structure of
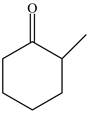
Figure 1
In the compound,
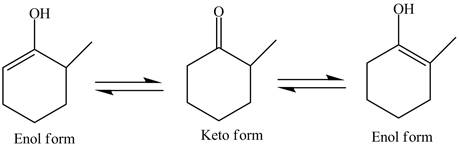
Figure 2
The compound
The enol structures of the compound
(b)
Interpretation:
The enol structure of the compound
Concept introduction:
Ketone also exists in two forms which are commonly known as keto-enol tautomerism. Tautomers are isomers which differ only in the position of the protons and electrons of the double bond of the electronegative atom in the compound. There is no change in the carbon skeleton of the compound. This phenomenon which involves simple proton transfer in an intramolecular fashion is known as tautomerism.
Answer to Problem 22.7P
The enol structure of the compound
Explanation of Solution
The structure of given compound
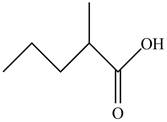
Figure 3
In the compound,
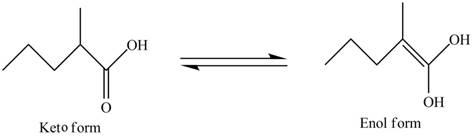
Figure 4
The enol structure of the compound
(c)
Interpretation:
The enol structure of the compound benzaldehyde is to be drawn. The explanation as to why the compound does not form enol structure.
Concept introduction:
The carbonyl compound contains a
Answer to Problem 22.7P
There is no
Explanation of Solution
The given compound benzaldehydeis shown below.
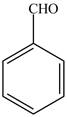
Figure 5
In the compoundbenzaldehydethere is no
In benzaldehyde, due to the absence of
(d)
Interpretation:
The enol structure of the compound
Concept introduction:
Ketone also exists in two forms which are commonly known as keto-enol tautomerism. Tautomers are isomers which differ only in the position of the protons and electrons of the double bond of the electronegative atom in the compound. There is no change in the carbon skeleton of the compound. This phenomenon which involves simple proton transfer in an intramolecular fashion is known as tautomerism.
Answer to Problem 22.7P
The enol structure of the compound
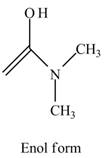
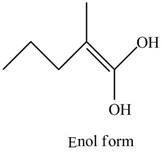
Explanation of Solution
The structure of the given compound
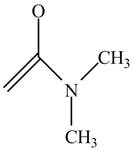
Figure 6
In the compound,
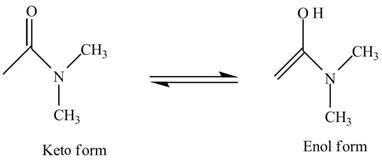
Figure 7
The enol structure of the compound
(e)
Interpretation:
The enol structure of the compound
Concept introduction:
Ketone also exists in two forms which are commonly known as keto-enol tautomerism. Tautomers are isomers which differ only in the position of the protons and electrons of the double bond of the electronegative atom in the compound. There is no change in the carbon skeleton of the compound. This phenomenon which involves simple proton transfer in an intramolecular fashion is known as tautomerism.
Answer to Problem 22.7P
There is no
Explanation of Solution
The given compound
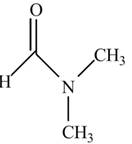
Figure 8
In the compound
In
Want to see more full solutions like this?
Chapter 22 Solutions
EBK ORGANIC CHEMISTRY
- 1. Draw structures for the following: (a) N,N-dimethylpentanamide (b) Acetamide(c) 2,3-dimethylpentanamide (d) N-ethylbenzamidearrow_forwardDraw line structures of the following compounds and the product you would obtain from the reduction of each.(a) Isopropyl methyl ketone (b) p-Hydroxybenzaldehyde(c) 2-Methylcyclopentanonearrow_forward(a) Explain why phentermine [PhCH2C(CH3)2NH2] can’t be made by a reductive amination reaction.(b) Give a systematic name for phentermine, one of the components of the banned diet drug fen–phen.arrow_forward
- Draw a structural formula for the product formed by treating butanal with each reagent. (a) LiA1H4LiA1H4 followed by H2OH2O (b) NaBH4NaBH4 in CH3OH/H2O (c) H2/Pt (d) Ag(NH3)2+in NH3/H2O (e) H2CrO4, heat (f) HOCH2CH2OH,HClarrow_forwardArrange the members of each group in order of decreasing basicity: (a) Ammonia, aniline, methylamine (b) Acetanilide, aniline, N-methylaniline (c) 2,4-Dichloroaniline, 2,4-dimethylaniline, 2,4-dinitroaniline (d) 3,4-Dichloroaniline, 4-chloro-2-nitroaniline, 4-chloro-3-nitroaniline (e) Dimethylamine, diphenylamine, N-methylanilinearrow_forwardDraw the structure of each compound.(a) o-nitroanisole (b) 2,4-dimethoxyphenol (c) p-aminobenzoic acid(d) 4-nitroanilinearrow_forward
- Which, if any, of the following compounds can be prepared by a malonic ester synthesis? Show the alkyl halide you would use in each case. (a) Ethyl pentanoate (c) Ethyl 2-rnethylbutanoate (d) Ethyl 2,2-dimethylpropanoate (b) Ethyl 3-methylbutanoatearrow_forwardShow how you might utilize the reduction of an amide, oxime, or a nitrile to carry out each of the following transformations (a)Benzoic acid to N-ethyl-N-benzylamine (b)1-Bromopentane to hexylamine (c)Propanoic acid to tripropylamine (d)2-Butanone to sec-butylaminearrow_forward2. a) Draw the following: (a) nonanamide (b) N-methyloctanamide (c) (d) N-ethyl-N-propylpropanamide N-ethyl-2,4,6-trimethyldecanamidearrow_forward
- Draw the structure of the following: (a) p-chlorobenzoic anhydride (b) cis-3-Methylcyclohexanecarbonyl bromide (c) Ethyl 3-methylpentanoate (d) 3-chloro-N-ethyl-N-methylbenzamidearrow_forwardWrite a structural formula for each of the following compounds: (a) m-Chlorobenzoyl chloride (b) Trifluoroacetic anhydride (c) cis-1,2-Cyclopropanedicarboxylic anhydride (d) Ethyl cycloheptanecarboxylate (e) 1-Phenylethyl acetate (f) 2-Phenylethyl acetate (g) p-Ethylbenzamide (h) N-Ethylbenzamide (i) 2-Methylhexanenitrilearrow_forward4. Draw structures corresponding to the following names: (a) 2,2-Dimethylpropanoyl chloride (b) N-Methylbenzamide (c) 5,5-Dimethylhexanenitrile (d) tert-Butyl butanoate (e) trans-2-Methylcyclohexanecarboxamide ( f) p-Methylbenzoic anhydridearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





