
(a)
Interpretation:
The preparation of the 1-bromo-3-nitrobenzene from benzene or toluene or phenol has to be shown.
Concept Introduction:
Activating and deactivating groups:
The effect of substituents on the reaction rate of
Activating groups – ortho/para directing groups. The
Deactivating groups – metadirecting groups. The rate of reaction is decreased by a deactivating groups (electron withdrawing groups) relative to hydrogen.
(a)
Explanation of Solution
Given target compound,
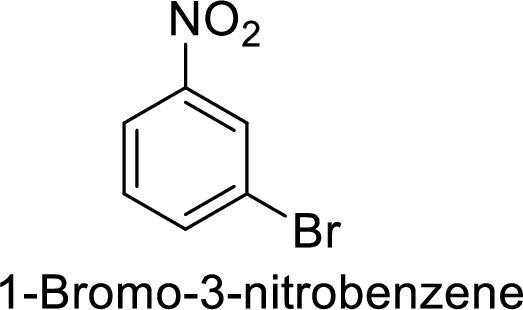
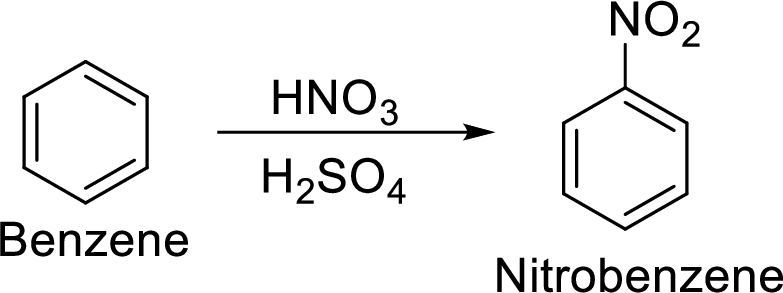
Step 1: Nitration of benzene ring by the reaction of benzene with nitrating mixture.
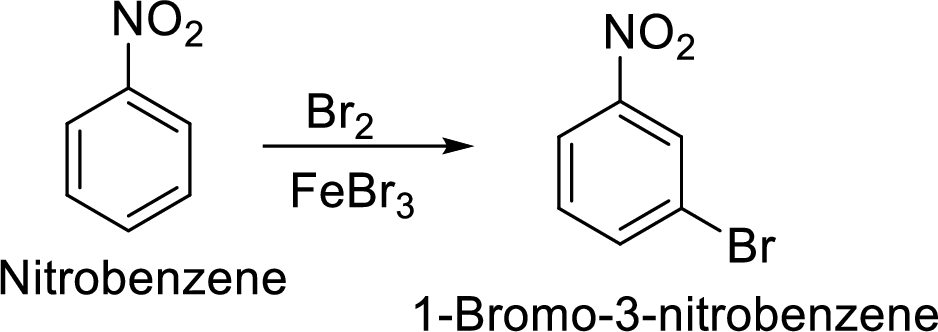
Step 2: Halogenation of nitrobenzene by the reaction of nitrobenzene with bromine in the presence of Lewis acid.
(b)
Interpretation:
The preparation of the 1-bromo-4-nitrobenzene from benzene or toluene or phenol has to be shown.
Concept Introduction:
Activating and deactivating groups:
The effect of substituents on the reaction rate of aromatic electrophilic substitution is given by activating or deactivating groups.
Activating groups – ortho/para directing groups. The rate of reaction is increased by an activating groups (electron donating groups) relative to hydrogen.
Deactivating groups – metadirecting groups. The rate of reaction is decreased by a deactivating groups (electron withdrawing groups) relative to hydrogen.
(b)
Explanation of Solution
Given target compound,
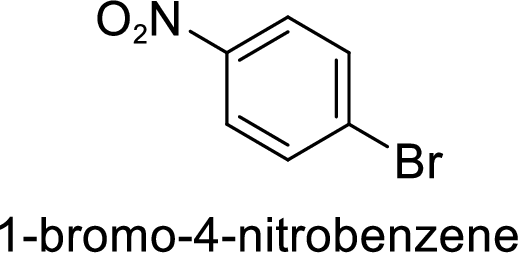
Step 1: Halogenation of benzene by the reaction of benzene with bromine in the presence of Lewis acid.
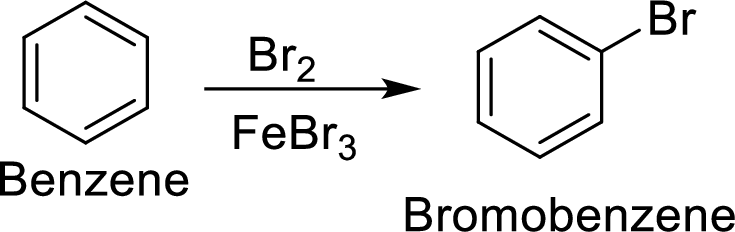
Step 2: Nitration of bromobenzene by the reaction of bromobenzene with nitrating mixture.
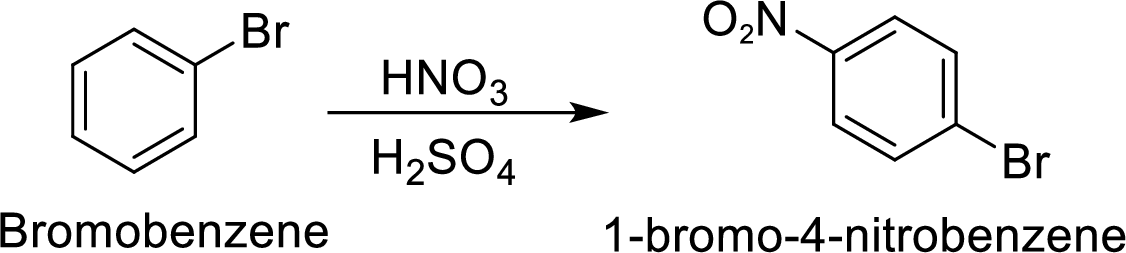
(c)
Interpretation:
The preparation of the 2,4-6-trinitrotoluene from benzene or toluene or phenol has to be shown.
Concept Introduction:
Activating and deactivating groups:
The effect of substituents on the reaction rate of aromatic electrophilic substitution is given by activating or deactivating groups.
Activating groups – ortho/para directing groups. The rate of reaction is increased by an activating groups (electron donating groups) relative to hydrogen.
Deactivating groups – metadirecting groups. The rate of reaction is decreased by a deactivating groups (electron withdrawing groups) relative to hydrogen.
(c)
Explanation of Solution
Given target compound,
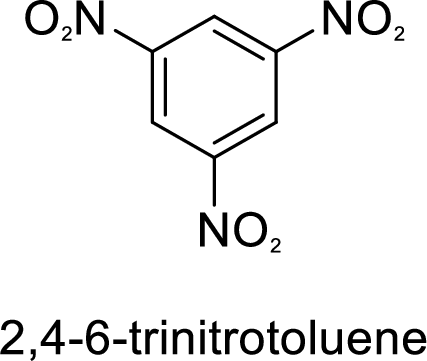
Methyl group in toluene is an activating and ortho-para directing group, so three times nitration of toluene gives the target compound.
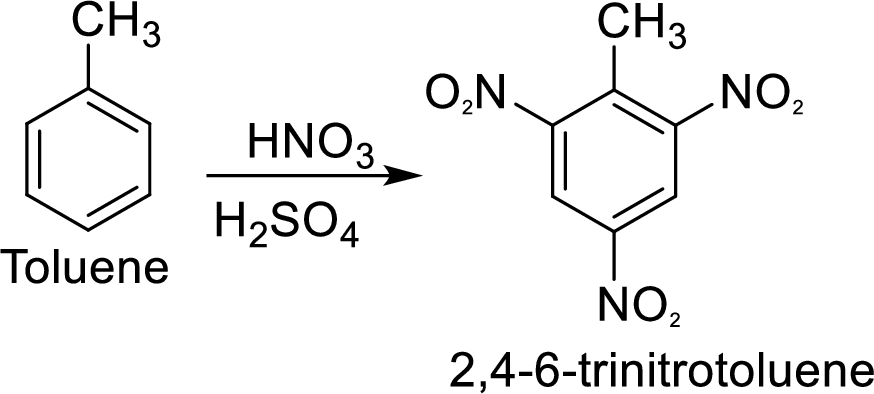
(d)
Interpretation:
The preparation of the m-chlorobenzoic acid from benzene or toluene or phenol has to be shown.
Concept Introduction:
Activating and deactivating groups:
The effect of substituents on the reaction rate of aromatic electrophilic substitution is given by activating or deactivating groups.
Activating groups – ortho/para directing groups. The rate of reaction is increased by an activating groups (electron donating groups) relative to hydrogen.
Deactivating groups – metadirecting groups. The rate of reaction is decreased by a deactivating groups (electron withdrawing groups) relative to hydrogen.
(d)
Explanation of Solution
Given target compound,
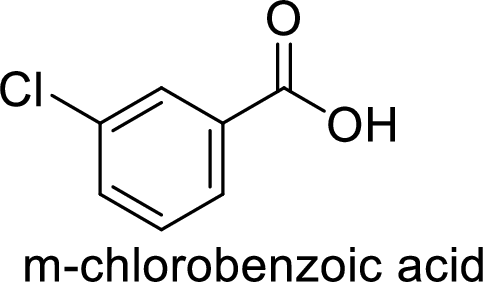
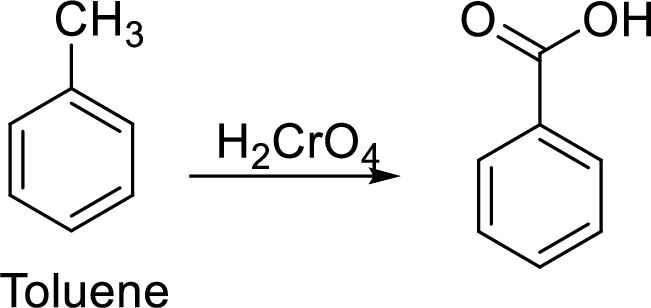
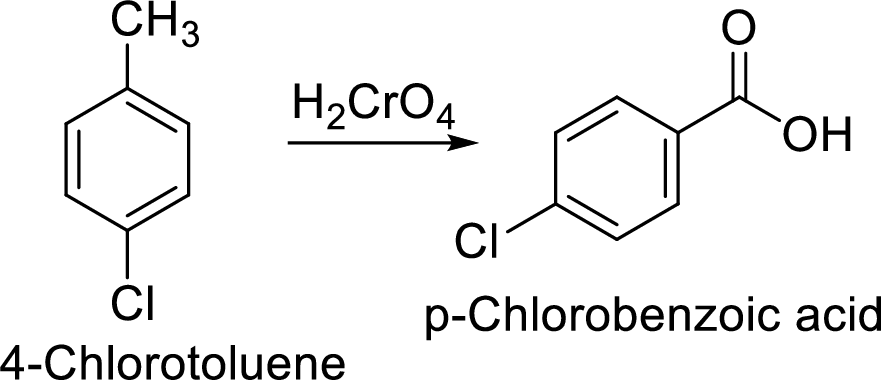
Step 1: Oxidation of toluene with chromic acid gives the benzoic acid.
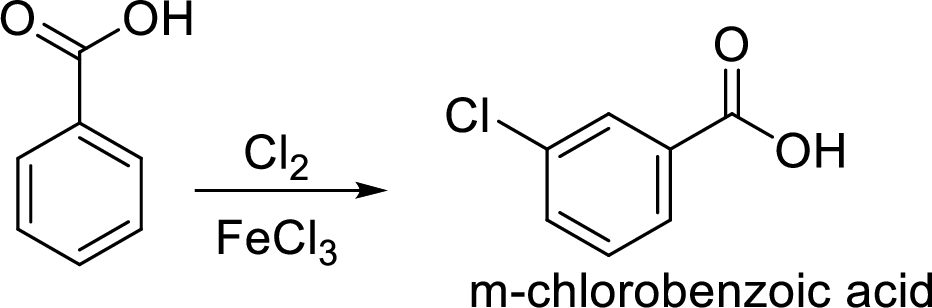
Step 2: Carboxyl group is a deactivating and meta directing group so the chlorination takes place at the meta position.
(e)
Interpretation:
The preparation of the p-chlorobenzoic acid from benzene or toluene or phenol has to be shown.
Concept Introduction:
Activating and deactivating groups:
The effect of substituents on the reaction rate of aromatic electrophilic substitution is given by activating or deactivating groups.
Activating groups – ortho/para directing groups. The rate of reaction is increased by an activating groups (electron donating groups) relative to hydrogen.
Deactivating groups – metadirecting groups. The rate of reaction is decreased by a deactivating groups (electron withdrawing groups) relative to hydrogen.
(e)
Explanation of Solution
Given target compound,
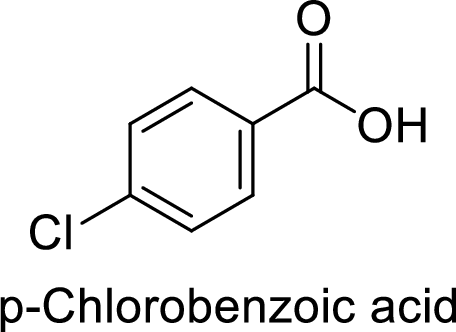
Step 1: Halogenation of toluene by the reaction of toluene with chlorine in the presence of Lewis acid.
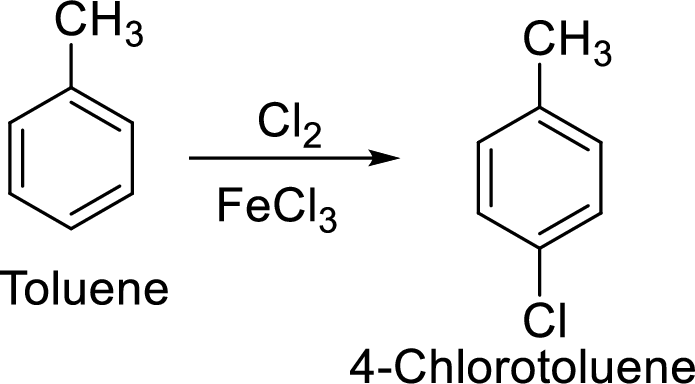
Step 2: Oxidation of 4-chlorotoluene by chromic acid gives the target compound.
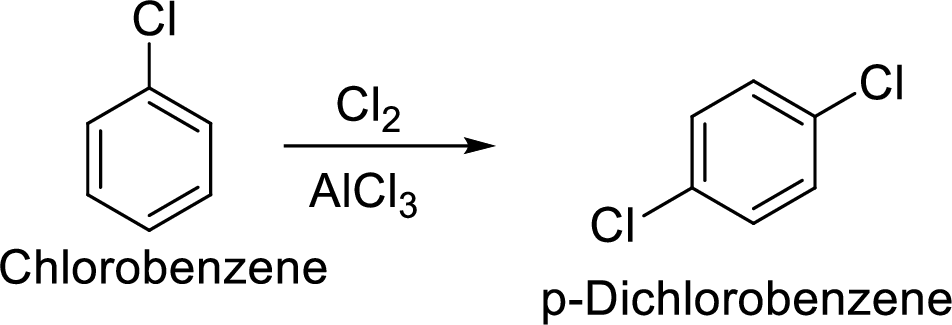
(f)
Interpretation:
The preparation of the p-dichlorobenzene from benzene or toluene or phenol has to be shown.
Concept Introduction:
Activating and deactivating groups:
The effect of substituents on the reaction rate of aromatic electrophilic substitution is given by activating or deactivating groups.
Activating groups – ortho/para directing groups. The rate of reaction is increased by an activating groups (electron donating groups) relative to hydrogen.
Deactivating groups – metadirecting groups. The rate of reaction is decreased by a deactivating groups (electron withdrawing groups) relative to hydrogen.
(f)
Explanation of Solution
Given target compound,
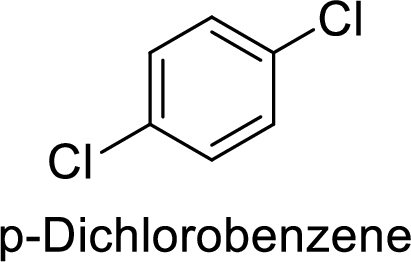
Step 1: Halogenation of benzene by the reaction of benzene with chlorine in the presence of Lewis acid.
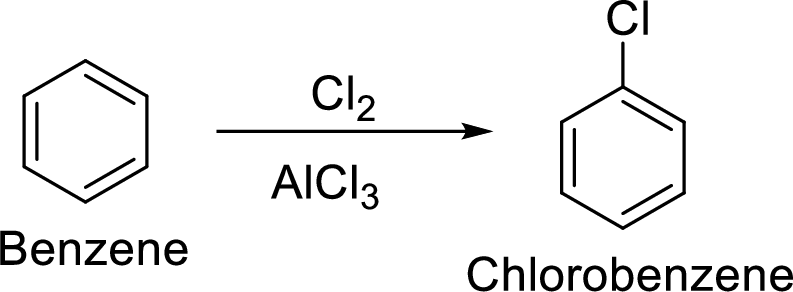
Step 2: Second halogenation of chlorobenzene by the reaction of chlorobenzene with chlorine in the presence of Lewis acid.
(g)
Interpretation:
The preparation of the 1-bromo-4-nitrobenzene from benzene or toluene or phenol has to be shown.
Concept Introduction:
Activating and deactivating groups:
The effect of substituents on the reaction rate of aromatic electrophilic substitution is given by activating or deactivating groups.
Activating groups – ortho/para directing groups. The rate of reaction is increased by an activating groups (electron donating groups) relative to hydrogen.
Deactivating groups – metadirecting groups. The rate of reaction is decreased by a deactivating groups (electron withdrawing groups) relative to hydrogen.
(g)
Explanation of Solution
Given target compound,
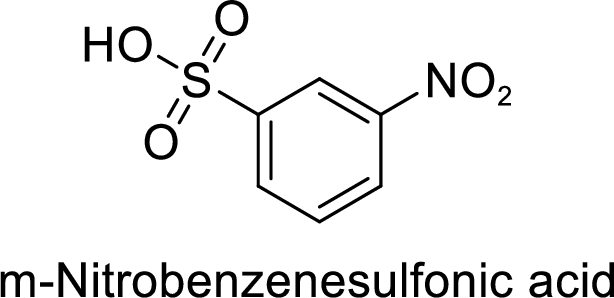
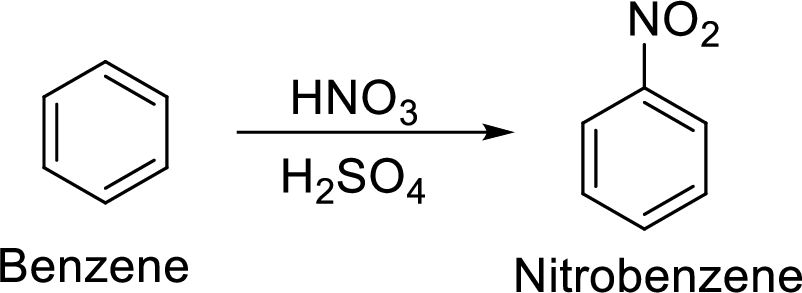
Step 1: Nitration of benzene by the reaction of benzene with nitrating mixture.
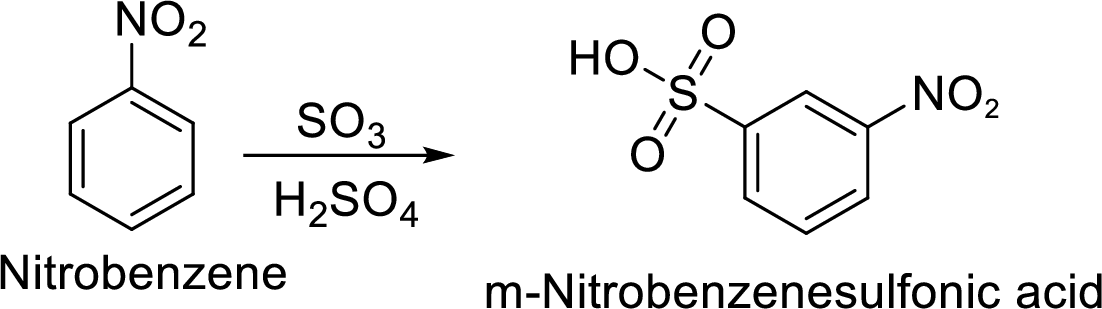
Step 2: Sulfonation of nitrobenzene by the reaction with
Want to see more full solutions like this?
Chapter 22 Solutions
ORGANIC CHEMISTRY-OWL V2 ACCESS
- Show how you would synthesize the following compounds, starting with benzene or toluene and any necessary acyclic reagents. Assume para is the major product (and separable from ortho) in ortho, para mixtures. (a) 3-nitro-4-bromobenzoic acid (b) 3-nitro-5-bromobenzoic acid (c) 4-butylphenol (d) 2-(4-methylphenyl)butan-2-olarrow_forwardStarting with benzene, toluene, or phenol as the only sources of aromatic rings, show how to synthesize the following. Assume in all syntheses that mixtures of ortho-para products can be separated into the desired isomer. Q.) 1-Bromo-4-nitrobenzenearrow_forwardGive reasons for the following: (i) p-nitrophenol is more acidic than p-methylphenol. (ii) Bond length of C—O bond in phenol is shorter than that in methanol. (iii) (CH3)3C—Br on reaction with sodium methoxide (Na+ _OCH3) gives alkene as the main product and not an ether.arrow_forward
- 4) Aromatic compounds are among the most abundant and versatile in nature. From a synthetic point of view, these compounds, despite their stabilities, are quite useful and can undergo reactions under special conditions and by specific mechanisms, such as the Electrophilic Aromatic Substitution (SAE) and the Nucleophilic Aromatic Substitution (SNAr). Based on this, please answer the following items: (b) How would you prepare the following compounds starting from benzene? Explain the second in a different wayarrow_forwardShow how you would synthesize the following compounds, starting with benzene or toluene and any necessary acyclic reagents. Assume para is the major product (and separable from ortho) in ortho, para mixtures.(a) ethoxybenzene (b) 1,2-dichloro-4-nitrobenzene (c) 1-phenylpropan-2-olarrow_forwardStarting with benzene, toluene, or phenol as the only sources of aromatic rings, show how to synthesize the following. Assume in all syntheses that mixtures of ortho-para products can be separated into the desired isomer. Q.)2,4,6-Trinitrotoluene (TNT)arrow_forward
- (a) The Friedel-Crafts reaction of benzene with 2-chloro-3-methylbutane in the presence of AlCl3 occurs with a carbocation rearrangement. Give mechanistic explanation and the product formed. (b) Predict the product(s) will be formed from the following reactions: (i) Bromination of p-methylbenzoic acid (ii) Sulphonation of m-bromoanisole (iii) Friedel-craft acylation of o-bromonitrobenzenearrow_forward(a) Account for the following :(i) Electrophilic substitution reactions in haloarenes occur slowly.(ii) Haloalkanes, though polar, are insoluble in water.(b) Arrange the following compounds in increasing order of reactivity towards SN2 displacement:2-Bromo-2-Methylbutane, 1-Bromopentane, 2-Bromopentanearrow_forwardShow, by writing an appropriate series of equations, how you could prepare propyne from each of the following compounds as starting materials. You may use any necessary organic or inorganic reagents. (a) 2-Propanol (d) 1,1-Dichloroethane (b) 1-Propanol (e) Ethyl alcohol (c) Isopropyl bromidearrow_forward
- Predict the major products (including stereochemistry) when cis-3-methylcyclohexanol reacts with the following reagents. (a) concentrated HBr (b) TsCl/pyridine, then NaBrarrow_forward(a) Draw the structure of the following :(i) p-Methylbenzaldehyde (ii) 4-Methylpent-3-en-2-one(b) Give chemical tests to distinguish between the following pairs of compounds :(i) Benzoic acid and Ethyl benzoate, (ii) Benzaldehyde and Acetophenone.(iii) Phenol and Benzoic acid.arrow_forwardSpecify reagents suitable for converting 3-ethyl-2-pentene to each of the following: (a) 2,3-Dibromo-3-ethylpentane (b) 3-Chloro-3-ethylpentane (c) 3-Ethyl-3-pentanol (d) 3-Ethyl-2-pentanol (e) 2,3-Epoxy-3-ethylpentane (f) 3-Ethylpentanearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





