
Concept explainers
(a)
Interpretation:
The structure of diethyl malonate showing acidic hydrogens is to be stated. The reason as to why it is more acidic than ordinary ester is to be stated.
Concept introduction:
Acidic hydrogen is defined as hydrogen that carries positive charge when the acid dissociates. The acidity in esters depends upon the stability of enolate ion. The higher is the stability of enolate ion higher is the acidity.
Answer to Problem 22.1P
The structure of the diethyl malonate showing acidic hydrogens is given below.
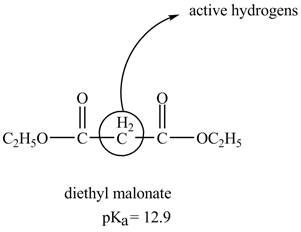
The diethyl malonate is more acidic than ordinary ester because its conjugate base is stabilized by delocalization of negative charge as shown below.
Explanation of Solution
In diethyl malonate the methylene group is surrounded by two carbonyl groups as shown below.
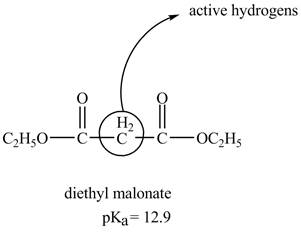
Figure 1
This active hydrogen is abstracted by base and generates a negative charge. The negative charge is delocalized between carbon and oxygen. This delocalization stabilizes the enolate ion. However, the presence of two carbonyl group increases the polar effect and stabilize the enolate ion. The diethyl malonate is more acidic than ordinary ester as its conjugate base is stabilized by delocalization of negative charge.
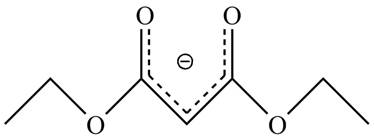
The acidity of the carbonyl compound is directly proportional to the stability of the enolate ion. The conjugate base stabilized by delocalization of diethyl malonate is shown below.
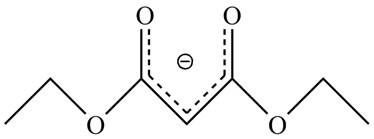
Figure 2
The structure of the diethyl malonate showing acidic hydrogens is given in Figure 2. The diethyl malonate is more acidic than ordinary ester due to delocalization of negative charge in its conjugate base.
(b)
Interpretation:
The structure of ethyl acetoacetate showing acidic hydrogens is to be stated. The reason as to why it is more acidic than ordinary ester is to be stated.
Concept introduction:
Acidic hydrogen is defined as hydrogen carry positive charge when the acid dissociates. The acidity in esters depends upon the stability of enolate ion. The higher is the stability of enolate ion higher is the acidity.
Answer to Problem 22.1P
The structure of ethyl acetoacetate showing acidic hydrogens is given below.
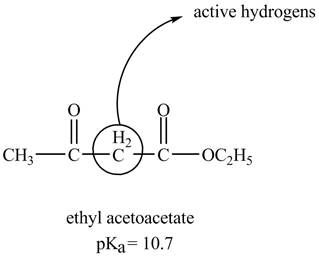
The ethyl acetoacetate is more acidic than ordinary ester because its conjugate base is stabilized by delocalization of negative charge as shown below.
Explanation of Solution
In ethyl acetoacetate, the methylene group is surrounded by two carbonyl groups as shown below.
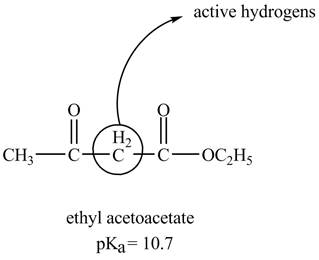
Figure 3
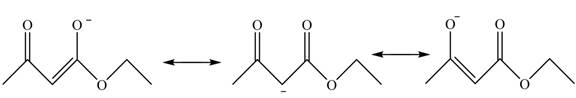
This active hydrogen is abstracted by base and generates a negative charge. The negative charge is delocalized between carbon and oxygen. This delocalization stabilizes the enolate ion. However, the presence of two carbonyl groups increases the polar effect and stabilize the enolate ions. The conjugate base stabilized by delocalization of ethyl acetoacetate is shown below.

Figure 4
The ethyl acetoacetate is more acidic than ordinary ester as its conjugate base is stabilized by delocalization of negative charge.
The structure of ethyl acetoacetate is shown in Figure 3 and the acidic character of ethyl acetoacetate is shown in Figure 4.
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry, Ebook And Single-course Homework Access
- Which of the following bases is strong enough to deprotonate N,Ndimethylacetamide [CH3CON(CH3)2, pKa = 30], so that equilibrium favors the products: (a) NaNH2; (b) NaOH?arrow_forwardWrite a structural formula for each of the following compounds: (a) m-Chlorobenzoyl chloride (b) Trifluoroacetic anhydride (c) cis-1,2-Cyclopropanedicarboxylic anhydride (d) Ethyl cycloheptanecarboxylate (e) 1-Phenylethyl acetate (f) 2-Phenylethyl acetate (g) p-Ethylbenzamide (h) N-Ethylbenzamide (i) 2-Methylhexanenitrilearrow_forwardGiven that C6H11COOH has a pKa = 4.8 and C6H11N+H3 has a pKa = 10.7, (a) What pH would you make the water layer to cause the carboxylic acid to dissolve in the water layer and the amine to dissolve in the ether layer? (b) What pH would you make the water layer to cause the carboxylic acid to dissolve in the ether layer and the amine to dissolve in the water layer?arrow_forward
- The odor of ripe bananas and many other fruits is due to the presence of esters. For example: Banana oil (isopentyl acetate) (a) Write the name (common or IUPAC) of the ester responsible for the fragrance of the following: pineapple, orange, apple, peach, & lavender (b) Choose one fragrant from (a) and name the alcohol and the carboxylic acid needed to synthesize this ester. (c) Show the detailed mechanism of the Fischer Esterification reaction that will be involved in the synthesis of the fragrant you have chosen in part (a).arrow_forwardPyridine forms a stronger Lewis acid-base complex with SO3 than SO2. However, pyridine forms a weaker complex with SF6 than with SF4. Explain the difference.arrow_forwardWhich, if any, of the following compounds can be prepared by a malonic ester synthesis? Show the alkyl halide you would use in each case. (a) Ethyl pentanoate (c) Ethyl 2-rnethylbutanoate (d) Ethyl 2,2-dimethylpropanoate (b) Ethyl 3-methylbutanoatearrow_forward
- Suggest a possible structure for Compound X.arrow_forwardUsing molecular structures, what will happen in the following situations: 1) Benzoic acid is dissolved in diethyl ether and then 1 M HCl (aq) is added: 2) Ethyl 4-aminobenzoate is dissolved in dichloromethane and then 1 M NaOH (aq) is addedarrow_forwardArrange the members of each group in order of decreasing basicity: (a) Ammonia, aniline, methylamine (b) Acetanilide, aniline, N-methylaniline (c) 2,4-Dichloroaniline, 2,4-dimethylaniline, 2,4-dinitroaniline (d) 3,4-Dichloroaniline, 4-chloro-2-nitroaniline, 4-chloro-3-nitroaniline (e) Dimethylamine, diphenylamine, N-methylanilinearrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


