
Concept explainers
Use the
Compound A Molecular formula:
Compound B Molecular formula:
Compound C Molecular formula:
Compound D Molecular formula:
Interpretation: The structure of each compound from the given
Concept introduction: Spectroscopy method is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton
Answer to Problem 21.74P
The structure of A compound from the given data of
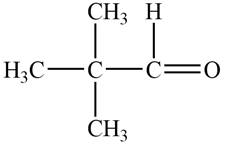
The structure of B compound from the given data of
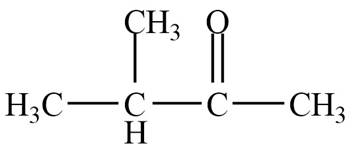
The structure of C compound from the given data of
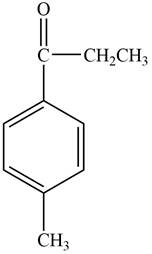
The structure of D compound from the given data of
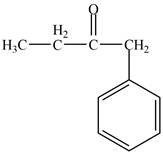
Explanation of Solution
Structure determination of Compound A:
The molecular formula of the Compound A is
Information from
The
The
Information from
The observed chemical shift value at
The observed chemical shift value at
Thus, the structure of Compound A from the given
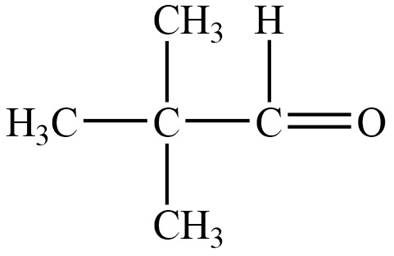
Figure 1
Structure determination of Compound B:
The molecular formula of the Compound B is
Information from
The
Information from
The observed chemical shift value at
The observed chemical shift value at
The observed chemical shift value at
Thus, the structure of Compound B from the given
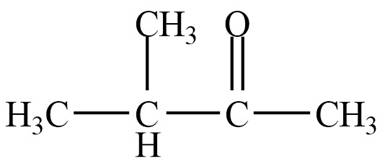
Figure 2
Structure determination of Compound C:
The molecular formula of the Compound C is
Information from
The
Information from
The observed chemical shift value at
The observed chemical shift value at
The observed chemical shift value at
The observed chemical shift values at
Thus, the structure of Compound C from the given
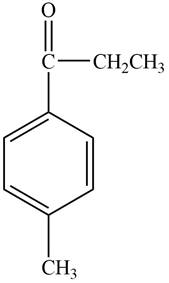
Figure 3
Structure determination of Compound D:
The molecular formula of the Compound D is
Information from
The
Information from
The observed chemical shift value at
The observed chemical shift value at
The observed chemical shift value at
The observed chemical shift value at
Thus, the structure of Compound D from the given
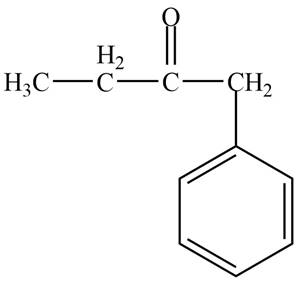
Figure 4
The structures of A, B, C, and D compounds from the given data of
Want to see more full solutions like this?
Chapter 21 Solutions
ORGANIC CHEMISTRY
- Ketones undergo a reduction when treated with sodium borohydride, NaBH4. What is the structure of the compound produced by reaction of 2-butanone with NaBH4 if it has an IR absorption at 3400 cm-1 and M+=74 in the mass spectrum?arrow_forward3-Chlorocyclopropene, on treatment with AgBF4, gives a precipitate of AgCl and a stable solution of a product that shows a single 1H NMR absorption at 11.04 δ. What is a likely structure for the products, and what is its relation to HĂ¼ckel’s rule?arrow_forwardCompound A has molecular formula C7H7X. Its 1H-NMR spectrum shows a singlet at 2.26 ppm and two doublets, one at 6.95 ppm and one at 7.28 ppm. The singlet has an integral of three and the doublets each have an integral of two. Its 13C-NMR shows five signals. The mass spectrum of A shows a peak at m/z = 170 and another peak at m/z = 172; the relative height of the two peaks is 1:1 respectively. - Identify what atom X is, explaining your reasoning - Identify Compound A, explaining your reasoning Compound A is treated with a mixture of nitric and sulfuric acids to generate Compound B. The 1H-NMR spectrum of B shows two singlets, one at 2.52 pm and one at 8.13 ppm. The 13C-NMR spectrum of B shows five signals. The mass spectrum of B shows a peak at m/z = 260 and another peak at m/z = 262; the relative height of the two peaks is 1:1 respectively. - Identify compound B, explaining your reasoning Compound B is treated with sodium ethoxide to generate compound C. The 1H-NMR spectrum of C shows…arrow_forward
- Compound A has molecular formula C7H7X. Its 1H-NMR spectrum shows a singlet at 2.25 ppm and two doublets, one at 7.28 ppm and one at 7.39 ppm. The singlet has an integral of three and the doublets each have an integral of two. The mass spectrum of A shows a peak at m/z = 126 and another peak at m/z = 128; the relative height of the two peaks is 3:1 respectively. - Identify what atom X is, explaining your reasoning - Identify Compound A, explaining your reasoningarrow_forwardAssume a compound with the formula C4H8O. a) How many double bonds and/or rings does your compound contain? b) If your compound shows an infrared absorption peak at 1715 cm-1, what functional group does it have? c) If your compound shows a single 1H NMR absorption peak at 2.1 δ, what is its structure?arrow_forwardIdentify the structures of isomers H and I (molecular formula C&HuN). a. Compound H: IR absorptions at 3365, 3284, 3026, 2932, 1603, and 1497 cm 2H 5 H 2H 2H 0 2 3 4 5 6 7 ppm b. Compound I: IR absorptions at 3367, 3286, 3027, 2962, 1604, and 1492 cm1 3 H 5 H 1 H 2 H 3 2 8 5 4 7arrow_forward
- Compound A, C8H10O, has the IR and 1H NMR spectra shown. Propose a structure consistent with the observed spectra, and label each peak in the NMR spectrum. Note that the absorption at 5.5 î disappears when D2O is added.arrow_forwardThe H1H1 NMR spectrum shown corresponds to an unknown compound with the molecular formula C6H10C6H10. There are no strong IR bands between 2100 and 2300 or 3250 and 3350 cm−1. Deduce and draw the structure of the molecule that corresponds to the spectrum.arrow_forwardIdentify the structures of isomers H and I (molecular formula C8H11N).a.Compound H: IR absorptions at 3365, 3284, 3026, 2932, 1603, and 1497 cm−1b.Compound I: IR absorptions at 3367, 3286, 3027, 2962, 1604, and 1492 cm−1arrow_forward
- Identify the structure of compound A (molecular formula C9H10O) from the 1H NMR and IR spectra given.arrow_forwardDraw the structure of molecular formula C8H10O that produced the 1H NMR spectra shown below. The IR spectrum does not show a broad absorbance at 3300 cm–1 or a strong absorbance at 1710 cm–1.arrow_forwardCompound X (molecular formula C10H120) was treated with NH2NH2, ¯OH to yield compound Y (molecular formula C10H14). Match the 1H NMR spectra of X and Y to the corresponding structures of X and Y. Compound NH2NH2 Compound 'H NMR of X 6 H OH Y 1 H 5H 8. 6. 4 ppm or H NMR of Y 6 H 2H 5H 1 H multiplet multiplet 8. 6. 4. 3. 1 nnm 2. 2. 3, O:arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

