
Concept explainers
Interpretation:
The factors that determines whether a given complex will be diamagnetic or paramagnetic has to be given.
Concept Introduction:
Diamagnetic:
The compounds in which all the electrons are paired in their orbitals are considered as diamagnetic compounds. Such compounds will be repelled by the magnetic field.
Paramagnetic:
If a compound consists of one or more unpaired electrons in their valence shell then it is known as paramagnetic compound. Such compounds will be attracted by the magnetic field.
Crystal field splitting:
When ligands approach the central metal atom then the field strength of the ligand splits the outermost valence d orbitals of the central metal atom into two set of orbitals such as
The following diagram shows the splitting in the octahedral complexes:
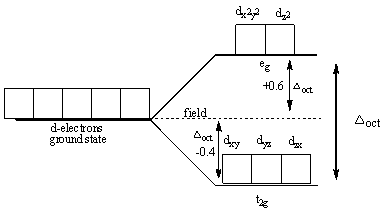
Splitting energy is the energy between
Pairing energy is the energy required to pair up electrons in an orbital. It is represented as
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
General Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





