
(a)
Interpretation:
Symbol of atom or ion has to be written that has 12 protons, 13 neutrons, and 10 electrons.
Concept Introduction:
Each and every element present in the Periodic table has a unique name. Some of the elements are named considering their
Chemical names are represented as atomic symbols. In the symbols, the mass number and atomic number are shown. The complete
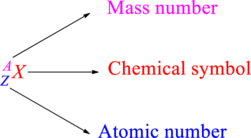
Atomic number is the total number of protons present in the atom of an element. Mass number is the total number of protons and neutrons present in nucleus of an atom.
(a)
Explanation of Solution
The species is said to contain 12 protons, 13 neutrons, and 10 electrons. Atomic number is equal to the number of protons and mass number is equal to the sum of protons and neutrons. Atomic number and mass number can be given as shown below.
The charge on the ion can be calculated considering the number of protons and electrons as shown below.
Element with the atomic number of 12 is magnesium. The atomic symbol of ion can be given as
(b)
Interpretation:
Symbol of atom or ion has to be written that has 13 protons, 14 neutrons, and 10 electrons.
Concept Introduction:
Refer part (a).
(b)
Explanation of Solution
The species is said to contain 13 protons, 14 neutrons, and 10 electrons. Atomic number is equal to the number of protons and mass number is equal to the sum of protons and neutrons. Atomic number and mass number can be given as shown below.
The charge on the ion can be calculated considering the number of protons and electrons as shown below.
Element with the atomic number of 13 is aluminium. The atomic symbol of ion can be given as
(c)
Interpretation:
Symbol of atom or ion has to be written that has 14 protons, 15 neutrons, and 14 electrons.
Concept Introduction:
Refer part (a).
(c)
Explanation of Solution
The species is said to contain 14 protons, 15 neutrons, and 14 electrons. Atomic number is equal to the number of protons and mass number is equal to the sum of protons and neutrons. Atomic number and mass number can be given as shown below.
The charge on the ion can be calculated considering the number of protons and electrons as shown below.
As the charge is zero, it is an atom and not an ion.
Element with the atomic number of 14 is silicon. The atomic symbol of atom can be given as
(d)
Interpretation:
Symbol of atom or ion has to be written that has 35 protons, 44 neutrons, and 36 electrons.
Concept Introduction:
Refer part (a).
(d)
Explanation of Solution
The species is said to contain 35 protons, 44 neutrons, and 36 electrons. Atomic number is equal to the number of protons and mass number is equal to the sum of protons and neutrons. Atomic number and mass number can be given as shown below.
The charge on the ion can be calculated considering the number of protons and electrons as shown below.
Element with the atomic number of 35 is bromine. The atomic symbol of ion can be given as
Want to see more full solutions like this?
Chapter 2 Solutions
Chemistry: Principles and Practice
- he vigorous reaction between aluminum and iodine gives the balanced equation: :math>2Al(s)+3I2(s)2AlI2(s). mg src=Images/HTML_99425-9-2QAP_image001.jpg alt="" align="top"/> at do the coefficients in this balanced chemical equation tell us about the proportions in which these substances react on a macroscopic (mole) basis?arrow_forwardThe element bromine is Br2, so the mass of a Br2 molecule is the sum of the mass of its two atoms. Bromine has two isotopes. The mass spectrum of Br2 produces three peaks with relative masses of 157.836, 159.834, and 161.832, and relative heights of 6.337, 12.499. and 6.164, respectively. (a) What isotopes of bromine are present in each of the three peaks? (b) What is the mass of each bromine isotope? (c) What is the average atomic mass of bromine? (d) What is the abundance of each of the two bromine isotopes?arrow_forwardTwo basic laws of chemistry are the law of conservation of mass and the law of constant composition. Which of these laws (if any) do the following statements illustrate? (a) Lavoisier found that when mercury(ll) oxide, HgO, decomposes, the total mass of mercury (Hg) and oxygen formed equals the mass of mercury(ll) oxide decomposed. (b) Analysis of the calcium carbonate found in the marble mined in Carrara, Italy, and in the stalactites of the Carlsbad Caverns in New Mexico gives the same value for the percentage of calcium in calcium carbonate. (c) Hydrogen occurs as a mixture of two isotopes, one of which is twice as heavy as the other.arrow_forward
- What evidence led to the conclusion that cathode rays had a negative charge?arrow_forwardIf mass Q/ mass A in AQ= 0.271 and mass Q/mass A in AxQy= 0.362, what is the formual of AxQy?arrow_forwardGive the number of atoms of the specified element in a formula unit of each of the following compounds, and calculate the molecular (formula) mass:(a) Hydrogen in ammonium benzoate, C6H5COONH4(b) Nitrogen in hydrazinium sulfate, N2H6SO4(c) Oxygen in the mineral leadhillite, Pb4SO4(CO3)2(OH)2arrow_forward
- What is the mass, in grams, of Vitamin C (C6H8O6) that contains 1.076 grams of carbon?arrow_forwardBoron, atomic number 5, occurs naturally as two isotopes, 10B and 11B, with natural abundances of 19.9% and 80.1%, respectively. (a) Will the mass percentage of F be the same in 10BF3 and 11BF3? If not, why is that the case?arrow_forwardGiven that you had 1 mol glucose (C 6H 12O 6), what would the mole ratio of glucose to oxygen be?arrow_forward
- The compound As2I4 is synthesized by reaction of arsenic metal with arsenic triiodide. If a solidcubic block of arsenic (d = 5.72 g/cm3) that is 3.00 cm on edge is allowed to react with 1.01 x 1024molecules of arsenic triiodide, what mass of As2I4 can be prepared? If the percent yield of As2I4 was75.6%, what mass of As2I4 was actually isolated?arrow_forwardWhat is the % molecular mass of Iron in Fe2S3?arrow_forwardA new compound has the empirical formula GaCl2. Thissurprises some chemists who, based on the position of gallium in the periodic table, expect a chloride of gallium to have the formula GaCl3 or possibly GaCl. They suggest that the “GaCl2” is really Ga[GaCl4], in which the bracketed group behaves as a unit with a -1 charge. Suggest experiments to test this hypothesisarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





