
Interpretation: The pressure difference across a simple U-tube manometer is to be calculated in the appropriate units.
Concept introduction: A simple U-tube Manometer is a device that can measure the pressure difference across a U-tube filled with fluids of different or the same density.
The pressure difference across the Manometer is given as,
In the above formula, the notations used are,
The density of the substance is given as,
Answer to Problem 2.1P
The pressure difference across a U-tube Manometer is 23544 N/m2.
Explanation of Solution
The U-tube Manometer mentioned in the question can be given as:
The fluid A is Mercury and the fluid B is Carbon tetrachloride here.
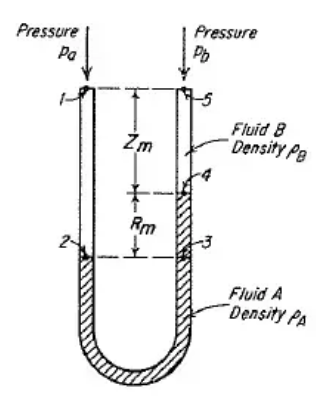
The given data is,
Substitute these values in equation (1) above,
The pressure difference across a U-tube Manometer for the given readings of density and manometer is 23544 N/m2.
Want to see more full solutions like this?
Chapter 2 Solutions
Unit Operations of Chemical Engineering
- (30) 6. In a process design, the following process streams must be cooled or heated: Stream No mCp Temperature In Temperature Out °C °C kW/°C 1 5 350 270 2 9 270 120 3 3 100 320 4 5 120 288 Use the MUMNE algorithm for heat exchanger networks with a minimum approach temperature of 20°C. (5) a. Determine the temperature interval diagram. (3) (2) (10) (10) b. Determine the cascade diagram, the pinch temperatures, and the minimum hot and cold utilities. c. Determine the minimum number of heat exchangers above and below the pinch. d. Determine a valid heat exchange network above the pinch. e. Determine a valid heat exchange network below the pinch.arrow_forwardUse this equation to solve it.arrow_forwardQ1: Consider the following transfer function G(s) 5e-s 15s +1 1. What is the study state gain 2. What is the time constant 3. What is the value of the output at the end if the input is a unit step 4. What is the output value if the input is an impulse function with amplitude equals to 3, at t=7 5. When the output will be 3.5 if the input is a unit steparrow_forward
- give me solution math not explinarrow_forwardExample (6): An evaporator is concentrating F kg/h at 311K of a 20wt% solution of NaOH to 50wt %. The saturated steam used for heating is at 399.3K. The pressure in the vapor space of the evaporator is 13.3 KPa abs. The 5:48 O Transcribed Image Text: Example (7): Determine thearrow_forward14.9. A forward feed double-effect vertical evaporator, with equal heating areas in each effect, is fed with 5 kg/s of a liquor of specific heat capacity of 4.18 kJ/kg K. and with no boiling point rise, so that 50 per cent of the feed liquor is evaporated. The overall heat transfer coefficient in the second effect is 75 per cent of that in the first effect. Steam is fed at 395 K and the boiling point in the second effect is 373 K. The feed is heated by an external heater to the boiling point in the first effect. It is decided to bleed off 0.25 kg/s of vapour from the vapour line to the second effect for use in another process. If the feed is still heated to the boiling point of the first effect by external means, what will be the change in steam consumption of the evaporator unit? For the purpose of calculation, the latent heat of the vapours and of the steam may both be taken as 2230 kJ/kgarrow_forward
- Example(3): It is desired to design a double effect evaporator for concentrating a certain caustic soda solution from 12.5wt% to 40wt%. The feed at 50°C enters the first evaporator at a rate of 2500kg/h. Steam at atmospheric pressure is being used for the said purpose. The second effect is operated under 600mmHg vacuum. If the overall heat transfer coefficients of the two stages are 1952 and 1220kcal/ m2.h.°C. respectively, determine the heat transfer area of each effect. The BPR will be considered and present for the both effect 5:49arrow_forwardالعنوان ose only Q Example (7): Determine the heating surface area 개 required for the production of 2.5kg/s of 50wt% NaOH solution from 15 wt% NaOH feed solution which entering at 100 oC to a single effect evaporator. The steam is available as saturated at 451.5K and the boiling point rise (boiling point evaluation) of 50wt% solution is 35K. the overall heat transfer coefficient is 2000 w/m²K. The pressure in the vapor space of the evaporator at atmospheric pressure. The solution has a specific heat of 4.18kJ/ kg.K. The enthalpy of vaporization under these condition is 2257kJ/kg Example (6): 5:48 An evaporator is concentrating F kg/h at 311K of a 20wt% solution of NaOH to 50wt %. The saturated steam used for heating is at 399.3K. The pressure in the vapor space of the evaporator is 13.3 KPa abs. The 5:48 1 J ۲/۱ ostrarrow_forwardExample 8: 900 Kg dry solid per hour is dried in a counter current continues dryer from 0.4 to 0.04 Kg H20/Kg wet solid moisture content. The wet solid enters the dryer at 25 °C and leaves at 55 °C. Fresh air at 25 °C and 0.01Kg vapor/Kg dry air is mixed with a part of the moist air leaving the dryer and heated to a temperature of 130 °C in a finned air heater and enters the dryer with 0.025 Kg/Kg alry air. Air leaving the dryer at 85 °C and have a humidity 0.055 Kg vaper/Kg dry air. At equilibrium the wet solid weight is 908 Kg solid per hour. *=0.0088 Calculate:- Heat loss from the dryer and the rate of fresh air. Take the specific heat of the solid and moisture are 980 and 4.18J/Kg.K respectively, A. =2500 KJ/Kg. Humid heat at 0.01 Kg vap/Kg dry=1.0238 KJ/Kg. "C. Humid heat at 0.055 Kg/Kg 1.1084 KJ/Kg. "C 5:42 Oarrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





