
Principles of Chemistry: A Molecular Approach (3rd Edition)
3rd Edition
ISBN: 9780321971944
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 19, Problem 58E
Interpretation Introduction
Interpretation:
The mass percent of 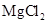 and the half life ofMg
and the half life ofMg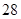 in a
in a 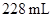 sample of an aqueous solutionis given. The decay rate of Mg
sample of an aqueous solutionis given. The decay rate of Mg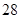 in the solution after
in the solution after 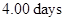 is to be calculated.
is to be calculated.
Concept introduction:
Decay constant is the quantity that expresses the rate of decrease of number of atoms of a radioactive element per second. Half life is defined as the time required for the number of nuclides to reach half of the original value.
To determine:The decay rate of Mg in the solution after
in the solution after 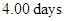
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Principles of Chemistry: A Molecular Approach (3rd Edition)
Ch. 19 - Prob. 19.1PCh. 19 - Prob. 19.2PCh. 19 - Prob. 19.3PCh. 19 - Prob. 19.4PCh. 19 - Prob. 19.5PCh. 19 - Prob. 19.6PCh. 19 - Prob. 19.7PCh. 19 - Prob. 1SAQCh. 19 - Prob. 2SAQCh. 19 - Prob. 3SAQ
Ch. 19 - Prob. 4SAQCh. 19 - Prob. 5SAQCh. 19 - Prob. 6SAQCh. 19 - Prob. 7SAQCh. 19 - Prob. 8SAQCh. 19 - Prob. 9SAQCh. 19 - Prob. 10SAQCh. 19 - Prob. 1ECh. 19 - Prob. 2ECh. 19 - Prob. 3ECh. 19 - Prob. 4ECh. 19 - Prob. 5ECh. 19 - Prob. 6ECh. 19 - Prob. 7ECh. 19 - Prob. 8ECh. 19 - Prob. 9ECh. 19 - Prob. 10ECh. 19 - Prob. 11ECh. 19 - Prob. 12ECh. 19 - Prob. 13ECh. 19 - Prob. 14ECh. 19 - Prob. 15ECh. 19 - Prob. 16ECh. 19 - Prob. 17ECh. 19 - Prob. 18ECh. 19 - Prob. 19ECh. 19 - Prob. 20ECh. 19 - Prob. 21ECh. 19 - Prob. 22ECh. 19 - Prob. 23ECh. 19 - Prob. 24ECh. 19 - Prob. 25ECh. 19 - Prob. 26ECh. 19 - Prob. 27ECh. 19 - Prob. 28ECh. 19 - Prob. 29ECh. 19 - Prob. 30ECh. 19 - Prob. 31ECh. 19 - Prob. 32ECh. 19 - Prob. 33ECh. 19 - Prob. 34ECh. 19 - Prob. 35ECh. 19 - Prob. 36ECh. 19 - Prob. 37ECh. 19 - Prob. 38ECh. 19 - Prob. 39ECh. 19 - Prob. 40ECh. 19 - Prob. 41ECh. 19 - Prob. 42ECh. 19 - Prob. 43ECh. 19 - Prob. 44ECh. 19 - Prob. 45ECh. 19 - Prob. 46ECh. 19 - Prob. 47ECh. 19 - Prob. 48ECh. 19 - Prob. 49ECh. 19 - Prob. 50ECh. 19 - Prob. 51ECh. 19 - Prob. 52ECh. 19 - Prob. 53ECh. 19 - Prob. 54ECh. 19 - Prob. 55ECh. 19 - Prob. 56ECh. 19 - Prob. 57ECh. 19 - Prob. 58ECh. 19 - Prob. 59ECh. 19 - Prob. 60ECh. 19 - Prob. 61ECh. 19 - Prob. 62ECh. 19 - Prob. 63ECh. 19 - Prob. 64ECh. 19 - Prob. 65ECh. 19 - Prob. 66ECh. 19 - Prob. 67ECh. 19 - Prob. 68ECh. 19 - Prob. 69E
Knowledge Booster
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY