
(a)
Interpretation:
The curved arrow mechanism for the given reaction is to be stated.
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 19.64AP
The curved arrow mechanism for the given reaction is shown below.
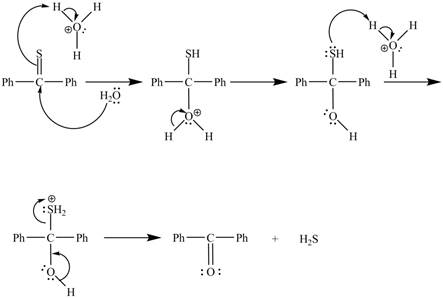
Explanation of Solution
The given reaction is shown below.
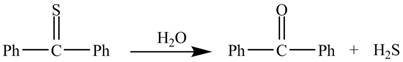
Figure 1
In the above reaction, diphenylmethanethione undergoes hydrolysis to yield benzophenone.
The curved arrow mechanism for the reaction is shown below.
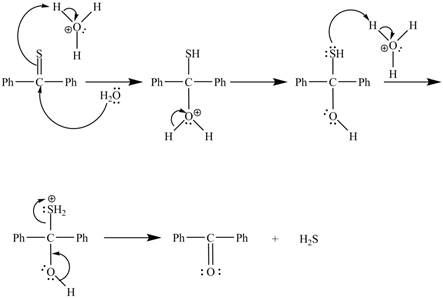
Figure 2
In the above mechanism, lone pairs of water attacks the
The curved arrow mechanism for the reaction is shown in Figure 2.
(b)
Interpretation:
The curved arrow mechanism for the given reaction is to be stated.
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 19.64AP
The curved arrow mechanism for the given reaction is shown below.
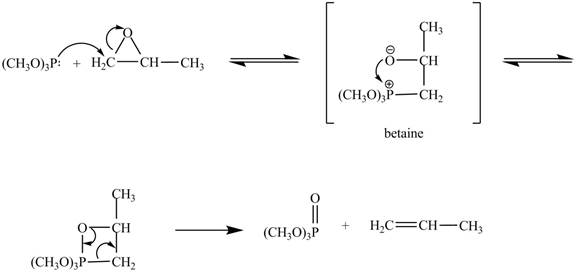
Explanation of Solution
The given reaction is shown below.

Figure 3
In the above reaction, trimethylphosphite reacts with
The curved arrow mechanism for the given reaction is shown below.
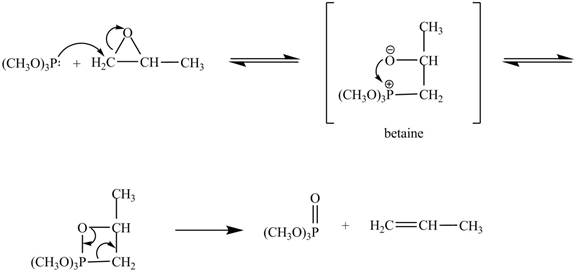
Figure 4
In the above mechanism, the trimethylphosphite reacts the least hindered site of the
The curved arrow mechanism for the given reaction is shown in Figure 4.
(c)
Interpretation:
The curved arrow mechanism for the given reaction is to be stated.
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 19.64AP
The curved arrow mechanism for the given reaction is shown below.
Explanation of Solution
The given reaction is shown below.
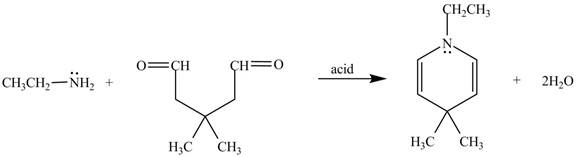
Figure 5
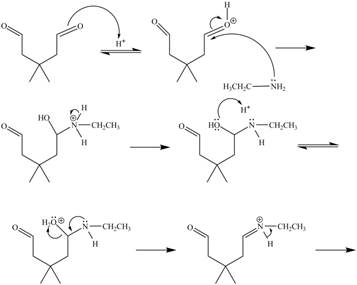
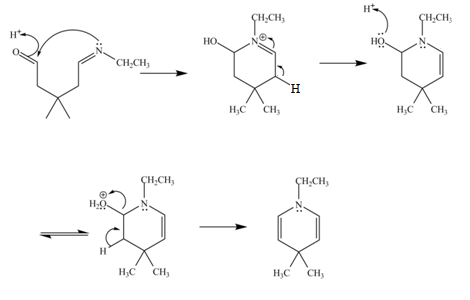
In the above reaction, ethylamine reacts with
The curved arrow mechanism for the given reaction is shown below.
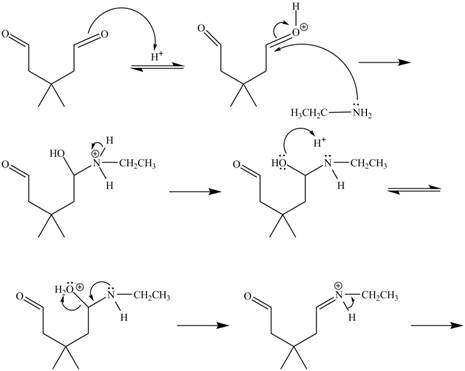

Figure 6
In the above mechanism, the carbonyl carbon of
The curved arrow mechanism for the given reaction is shown in Figure 6.
(d)
Interpretation:
The curved arrow mechanism for the given reaction is to be stated.
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 19.64AP
The curved arrow mechanism for the given reaction is shown below.
Explanation of Solution
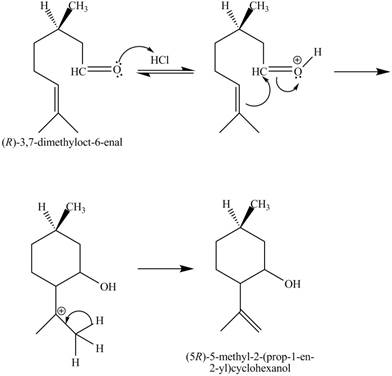
The given reaction is shown below.
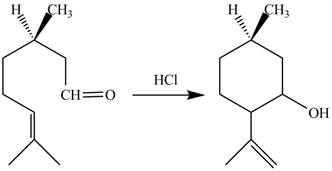
Figure 7
In the above reaction,
The curved arrow mechanim for the given reaction is shown below.
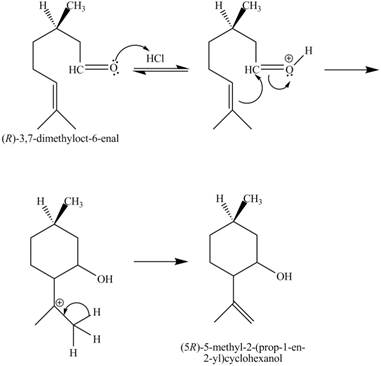
Figure 8
In the above mechanism, the carbonyl carbon of the compound
The curved arrow mechanism of the giveh reaction is shown in Figure 8.
(d)
Interpretation:
The curved arrow mechanism for the given reaction is to be stated.
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 19.64AP
The curved arrow mechanism for the given reaction is shown below.
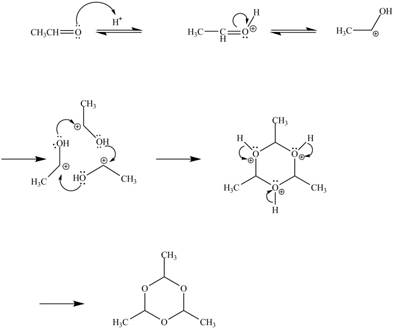
Explanation of Solution
The given reaction is shown below.
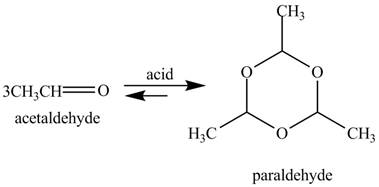
Figure 9
In the above reaction, acetaldehyde in the presence of acid catalyst yields a compound paraldehyde.
The curved arrow mechanism for the given reaction is shown below.
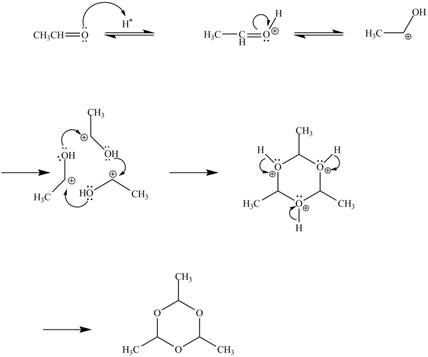
Figure 10
In the above mechanism, the acetaldehyde attacks the proton of acid catalyst to yield protonated acetaldehyde. The three moles of protonated acetaldehyde combines with each other to gives a cyclic structure. This cyclic structure on rearrangement gives the required product paraldehyde.
The curved arrow mechanism of the given reaction is shown in Figure 10.
Want to see more full solutions like this?
Chapter 19 Solutions
EBK ORGANIC CHEMISTRY
- (b) Suggest a reasonable biosynthesis for the naturally occurring alkaloid coniine (isolated from hemlock), starting from octanoic acid. Coniinearrow_forward(A) Provide the major organic product for the reaction below (B) Would the product be optically active of optically in active?arrow_forward(i) Explain why a high reaction temperature favours elimination reactions, instead of substitution reactions. (ii) Explain why polar aprotic solvents favour Sn2 reaction but not favour SN1 reaction.arrow_forward
- Outline syntheses of each of the following from aniline and any necessary organic or inorganic reagents. (a) p-Nitroaniline (b) 2,4-Dinitroaniline (c) p-Aminoacetanilidearrow_forward(a) Highlight four significant reasons why porphyrin derivatives are a popular Ochoice as photosensitizers in photo- thermal cancer therapy.arrow_forwardThe natural product halomon could theoretically arise from another naturally occurring compound known as myrcene. To accomplish this, a biochemical process that could deliver the synthetic equivalent of BrCi to all three double bonds would be required. (Chem Comm. 2014, 50, 13725) (a) Using three molar equivalents of BrCL please provide a mechanism to account for the formation of the bracketed structure (you do not need to show stereochemistry in this mechanism) HB (3 equiv) myrcene balomon 8.61a Add curved arrow(s) to show the mechanism steps. Edit Drawing sitsarrow_forward
- Provide the reagents and solvents (where appropriate) needed to bring about the following transformations. (a) CI (b)arrow_forwardCompare Electrophilic aromatic substitution reactivity to quinoline and isoquinoline. Please, explain with diagramsarrow_forward(b) Distinguish each as aromatic or antiaromatic in terms of electronic basis. Explain which one is more stable. (i) Compound A Compound B (ii) Compound C Compound D (iii) Compound E Compound Farrow_forward
- the following reaction scheme leads to the formation of compound C. give the structure of the final product C and of the intermediate products A and B and justify, using the mechanism, the formation of the product A. Give the serereochemistry of the final product obtainedarrow_forwardAnswer all parts of this question. (a) Give systematic names for the heterocycles shown below. CI Place the following molecules into order from most to least basic. detailed reasons for your answer. (b) NH3 N. H. S.arrow_forwardAccount for the following:(i) Aniline does not give Friedel-Crafts reaction.(ii) Ethylamine is soluble in water whereas aniline is not.(iii) pKb of methylamine is less than that of aniline.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
