
Concept explainers
(a)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
The C atom in the
Answer to Problem 19.1P
The product of the given reaction is
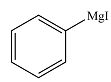
Explanation of Solution
The given reaction is
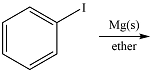
Magnesium is an active metal and reacts with the polar
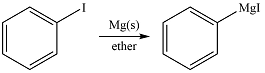
Thus, the product of the reaction is phenyl magensium iodide:

The structure of the product was drawn based on reversal of the charge on the carbon atom from the
(b)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
The C atom in the
Answer to Problem 19.1P
The product of the given reaction is
![]()
Explanation of Solution
The given reaction is
![]()
The
![]()
In essence, the reaction inserts the metal atom between the C and Br atoms and leads to a reversal of the charge on the carbon atom.
Thus, the product of the reaction is
![]()
The product of the reaction was drawn based on the reversal of charge on the C atom bonded to bromine.
(c)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
The C atom in the
Answer to Problem 19.1P
The product of the reaction is
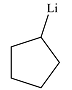
Explanation of Solution
The given reaction is
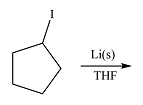
Li is a very active metal and will extract the iodine atom from the substrate. The
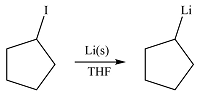
Effectively, the reaction results in the replacement of the iodine atom by Li atom, therefore, the product will be

The product of the reaction was drawn based on the reversal of the charge on the carbon atom initially bonded to iodine.
(d)
Interpretation:
The product of the given reaction is to be drawn.
Concept introduction:
The C atom in the
This reverses the initial polarity (charge) of the carbon, allowing it to form a bond with an electron-poor carbon from say a carbonyl carbon.
Answer to Problem 19.1P
The product of the given reaction is
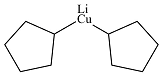
Explanation of Solution
The given reaction is
![]()
The product from part (c) is cyclopentyllithium. Two moles of this compound will react with one of copper(I) iodide to form
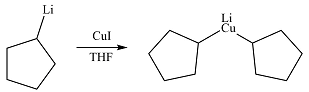
The carbon atom in the
The product of the reactio is, therefore,

The product of the reaction was drawn based formation of a bond between the relatively electron-rich carbon atoms and the copper atom.
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry: Principles And Mechanisms
- Draw the mechanism of the reaction.arrow_forward9. Draw all of the possible Monochlorination Products that would Result From the Free Radical Chlormation OF 23,4-TRIMethyl Pentane b. Calculate the To Yield For the major • Product given the Following Relative Restritus For 1° 2° and 30 Hydrogens toward Free Radical Chloration 5.0: 38 : 1 30 2° 1° C. what would be the major product in the Free Radical brominator Of the Same Molecule. Explain your Reasoning.arrow_forwardWhat is the complete reaction mechanism for the chlorination of Ethane, C2H6?arrow_forward
- A 13C NMR spectrum is shown for a molecule with the molecular formula of C6H100. Draw the structure that best fits this data. 220 200 180 160 140 120100 80 60 40 20 Drawingarrow_forwardPlease help me figure out the blan areas with step by step calculations.arrow_forwardneeding help draw all of the possible monochlorination products that would result from the free radical chlorination of 2,3,4-trimethylpentanearrow_forward
- HAND DRAWarrow_forwardBased on the 1H NMR, 13C NMR, DEPT 135 NMR and DEPT 90 NMR, provide a reasoning step and arrive at the final structure of an unknown organic compound containing 7 carbons. Dept 135 shows peak to be positive at 128.62 and 13.63 Dept 135 shows peak to be negative at 130.28, 64.32, 30.62 and 19.10. Provide assignment for the provided structurearrow_forwardO Predict the 'H NMR integration ratio for the following structure. IV I. 3 H A II. 1 H III. 2 H IV. 3 H I. 3 H B II. O H III. 2 H IV. 3 H I. 3 H C II. 2 H III. 2 Harrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
