
Concept explainers
(a)
Interpretation:
An acceptable name for the following amine should be determined:
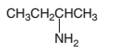
Concept Introduction:
Primary amines are named using the longest carbon chain bonded to the amine nitrogen, and suffix − amine is added at the end of the parent
The naming of secondary and tertiary amines that contains identical alkyl groups are done by using prefix di- or tri- followed by the name of primary amine. Secondary and tertiary amines are containing more than one kind of alkyl group are named as N-substituted primary amines.
Many different nitrogen heterocycles have different names based on ring types and ring size.
(b)
Interpretation:
An acceptable name for the following amine should be determined:
Concept Introduction:
Amines named are based on their structure (
Primary amines are named using the longest carbon chain bonded to the amine nitrogen, and suffix − amine is added at the end of the parent alkane removing the -e of it.
The naming of secondary and tertiary amines that contains identical alkyl groups are done by using prefix di- or tri- followed by the name of primary amine. Secondary and tertiary amines are containing more than one kind of alkyl group are named as N-substituted primary amines.
Many different nitrogen heterocycles have different names based on ring types and ring size.
(c)
Interpretation:
An acceptable name for the following amine should be determined:
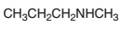
Concept Introduction:
Amines named are based on their structure (
Primary amines are named using the longest carbon chain bonded to the amine nitrogen, and suffix − amine is added at the end of the parent alkane removing the -e of it.
The naming of secondary and tertiary amines that contains identical alkyl groups are done by using prefix di- or tri- followed by the name of primary amine. Secondary and tertiary amines are containing more than one kind of alkyl group are named as N-substituted primary amines.
Many different nitrogen heterocycles have different names based on ring types and ring size.
(d)
Interpretation:
An acceptable name for the following amine should be determined:
Concept Introduction:
Amines named are based on their structure (
Primary amines are named using the longest carbon chain bonded to the amine nitrogen, and suffix − amine is added at the end of the parent alkane removing the -e of it.
The naming of secondary and tertiary amines that contains identical alkyl groups are done by using prefix di- or tri- followed by the name of primary amine. Secondary and tertiary amines are containing more than one kind of alkyl group are named as N-substituted primary amines.
Many different nitrogen heterocycles have different names based on ring types and ring size.
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
General, Organic, & Biological Chemistry
- The following molecule is a NH 3° amine 2° alcohol 1° alcohol 2° amine 1° amine 3° alcoholarrow_forwardWhat is the IUPAC name of a benzene ring with an amine group (NH2-) attached at carbon 1 and a bromine attached at carbon 2? O 2-bromoaniline O 2-bomotoluene O 2-bromobenzene O 2-bromophenolarrow_forwardDraw the products formed when each alcohol is oxidized with K 2Cr 2O 7. In some cases, no reaction occurs.arrow_forward
- What is the systematic IUPAC name for the given compound? CH3 CH3 CH;CHCH,CH,CH2 -Ń-CH3 a. N-methyl-4-methylhexan-1-amine b. 2,4-dimethylhexan-1-amine c. 2,2,N-trimethylpentan-1-amine d. N,N,4-trimethylpentan-1-aminearrow_forwardClassify each of the molecules below. CH3 CH3 1° amide 2° amide 3° amide not an amide at all || | NH -CH₂-CH3 CH3 C-N-CH2−NH— - CH3 1° amide 2° amide 3° amide not an amide at all CH3 -CH2−NH–CH3 1° amide 2° amide 3° amide not an amide at allarrow_forwardThe following amines have the same molecular formula (C5H13N), but their boiling points are significantly different. Explain why. H `NH2 2-Methylbutan-1-amine Boiling point = 97 °C N-Methylbutan-2-amine Boiling point = 84 °C N-Ethyl-N-methylethan-1-amine Boiling point = 65 °Carrow_forward
- Valacyclovir is an antiviral drug that slows the growth and spread of the herpes virus to help the body fight the infection. Shown below is the structure of the drug. Which of the following functional groups can be found in the structure of valacyclovir? Select one or more: Carboxylic Acid Alcohol Amide Benzene ооооооо Ester Alkyne Amine O Ether Hist HN H₂N NH₂arrow_forwardWhat products are formed when each alcohol is oxidized with K 2Cr 2O 7? In some cases, no reaction occurs.arrow_forwardName the following CH3CH2 1 CH3CCH₂CHCH3 11 CH3CH2 :N(CH3)2 O4,4-Diethyl-N,N-dimethylpentyl-2-amine O4,4-Diethyl-N,N-dimethylpentane-2-amine 4-Ethyl-4,N,N-trimethylhexane-2-amine 4-Ethyl-4,N,N-trimethylhexyl-2-aminearrow_forward
- Classify each amine as 1˚, 2˚, or 3˚. Give the IUPAC name for each amine.arrow_forwardName the following organic compound: CH₂ CH₂ H3C CH₂ OH O 2-oxo-5-pentanol O 4-oxo-1-pentanol O 5-hydroxy-2-pentanone O hydroxypentanone O 1-hydroxy-4-pentanonearrow_forwardWhat is the IUPAC name for the amine shown? CH3-CH2-CH-CH2-CH3 NH-CH3 What is the common name of the amine show? (do not include any spaces) CH3-CH2-N-CH,-CH, CH3 What is the common name for the amine shown? (do not include any spaces) N-CH,-CH3 CH3arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

